Dietary Alaska Pollack Protein Induces Acute Skeletal Muscle Hypertrophy in Rats, Regardless of Specific Amino Acid and Amino Acid Balance of Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Sources
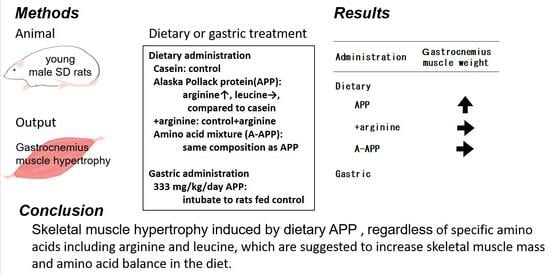
2.2. Animals and Experimental Design
2.3. Amino Acids Analysis in the Rat Serum
2.4. Statistical Analysis
3. Results
3.1. Effects on Final Body Weight, Body Weight Gain, and Total Food Intake (Table 3)
3.2. Effects of Gastrocnemius Muscle Weight
3.3. Effects on the Concentration of Free Amino Acids in Serum and Muscle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Koopman, R.; Ly, C.H.; Ryall, J.G. A metabolic link to skeletal muscle wasting and regeneration. Front. Physiol. 2014, 5, 32. [Google Scholar] [CrossRef]
- Chen, H.-C.; Lim, L.-M.; Chang, J.-M.; Misra, M. Save life and improve quality: Report from the 5th Congress of International Society for Hemodialysis. Hemodial. Int. 2014, 18, 163–171. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Layman, D.K. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J. Nutr. 1999, 129, 1102–1106. [Google Scholar] [CrossRef]
- Lim, C.H.; Gil, J.H.; Quan, H.; Viet, D.H.; Kim, C.K. Effect of 8-week leucine supplementation and resistance exercise training on muscle hypertrophy and satellite cell activation in rats. Physiol. Rep. 2018, 6, e13725. [Google Scholar] [CrossRef]
- Kitakaze, T.; Harada, N.; Imagita, H.; Yamaji, R. β-carotene increases muscle mass and hypertrophy in the soleus muscle in mice. J. Nutr. Sci. Vitaminol. 2015, 61, 481–487. [Google Scholar] [CrossRef]
- Hashimoto, R.; Sakai, A.; Murayama, M.; Ochi, A.; Abe, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Yanagawa, H.; Yasui, N.; et al. Effects of dietary soy protein on skeletal muscle volume and strength in humans with various physical activities. J. Med. Investig. 2015, 62, 177–183. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chiu, C.-Y.; Wang, L.-P.; Chiang, M.-T. Omega-3 Fatty Acids-Enriched Fish Oil Activates AMPK/PGC-1α Signaling and Prevents Obesity-Related Skeletal Muscle Wasting. Mar. Drugs 2019, 17, 380. [Google Scholar] [CrossRef]
- Le, N.H.; Kim, C.-S.; Park, T.; Park, J.H.Y.; Sung, M.-K.; Lee, D.G.; Hong, S.-M.; Choe, S.-Y.; Goto, T.; Kawada, T.; et al. Quercetin Protects against Obesity-Induced Skeletal Muscle Inflammation and Atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef]
- Rondanelli, M.; Rigon, C.; Perna, S.; Gasparri, C.; Iannello, G.; Akber, R.; Alalwan, T.A.; Freije, A.M. Novel Insights on Intake of Fish and Prevention of Sarcopenia: All Reasons for an Adequate Consumption. Nutrients 2020, 12, 307. [Google Scholar] [CrossRef]
- Mizushige, T.; Kawabata, F.; Uozumi, K.; Tsuji, T.; Kishida, T.; Ebihara, K. Fast-twitch muscle hypertrophy partly induces lipid accumulation inhibition with Alaska pollack protein intake in rats. Biomed. Res. 2010, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, F.; Mizushige, T.; Uozumi, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Kishida, T. Fish protein intake induces fast-muscle hypertrophy and reduces liver lipids and serum glucose levels in rats. Biosci. Biotechnol. Biochem. 2015, 79, 109–116. [Google Scholar] [CrossRef]
- Uchida, K.; Fujitani, M.; Mizushige, T.; Kawabata, F.; Hayamizu, K.; Uozumi, K.; Hara, Y.; Sawai, M.; Uehigashi, R.; Okada, S.; et al. Dietary Alaska pollack Protein Induces Acute and Sustainable Skeletal Muscle Hypertrophy in Rats. Nutrients 2022, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, M.; Mizushige, T.; Kawabata, F.; Uozumi, K.; Yasui, M.; Hayamizu, K.; Uchida, K.; Okada, S.; Keshab, B.; Kishida, T. Dietary Alaska pollack protein improves skeletal muscle weight recovery after immobilization-induced atrophy in rats. PLoS ONE 2019, 14, e0217917. [Google Scholar] [CrossRef] [PubMed]
- Morisasa, M.; Goto-Inoue, N.; Sato, T.; Machida, K.; Fujitani, M.; Kishida, T.; Uchida, K.; Mori, T. Investigation of the Lipid Changes That Occur in Hypertrophic Muscle Due to Fish Protein-Feeding Using Mass Spectrometry Imaging. J. Oleo Sci. 2019, 68, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Sabatini, D.M. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab. 2017, 26, 301–309. [Google Scholar] [CrossRef]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.-J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary L-Arginine Supplementation Reduces White Fat Gain and Enhances Skeletal Muscle and Brown Fat Masses in Diet-Induced Obese Rats. J. Nutr. 2009, 139, 230–237. [Google Scholar] [CrossRef]
- Gross, K.L.; Hartman, W.J.; Ronnenberg, A.; Prior, R.L. Arginine-Deficient Diets Alter Plasma and Tissue Amino Acids in Young and Aged Rats. J. Nutr. 1991, 121, 1591–1599. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Orally Administered Leucine Stimulates Protein Synthesis in Skeletal Muscle of Postabsorptive Rats in Association with Increased eIF4F Formation. J. Nutr. 2000, 130, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Diet, C.B.; Diet, U.; Diet, N.P. Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J. Nutr. 1977, 107, 1340–1348. [Google Scholar]
- Kurtz, J.A.; VanDusseldorp, T.A.; Doyle, J.A.; Otis, J.S. Taurine in sports and exercise. J. Int. Soc. Sports Nutr. 2021, 18, 1–20. [Google Scholar] [CrossRef]
- Arancibia-Garavilla, Y.; Toledo, F.; Casanello, P.; Sobrevia, L. Nitric Oxide Synthesis Requires Activity of the Cationic and Neutral Amino Acid Transport System y+ L in Human Umbilical vein Endothelium. Exp. Physiol. 2003, 88, 699–710. [Google Scholar] [CrossRef]
- Fujimoto, M.; Kameji, T.; Kanaya, A.; Hagihira, H. Purification and Properties of Rat Small Intestinal Arginase. J. Biochem. 1976, 79, 441–449. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Christofoletti, D.C.; Pezolato, V.A.; de Cassia Marqueti Durigan, R.; Prestes, J.; Tibana, R.A.; Pereira, E.C.; de Sousa Neto, I.V.; Durigan, J.L.; da Silva, C.A. Leucine minimizes denervation-induced skeletal muscle atrophy of rats through akt/mtor signaling pathways. Front. Physiol. 2015, 6, 73. [Google Scholar] [CrossRef]
- Pereira, M.G.; Silva, M.T.; da Cunha, F.M.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp. Gerontol. 2015, 72, 269–277. [Google Scholar] [CrossRef]
- Pereira, M.G.; Baptista, I.L.; Carlassara, E.O.C.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine Supplementation Improves Skeletal Muscle Regeneration after Cryolesion in Rats. PLoS ONE 2014, 9, e85283. [Google Scholar] [CrossRef]
- Savary-Auzeloux, I.; Magne, H.; Migné, C.; Oberli, M.; Breuillé, D.; Faure, M.; Vidal, K.; Perrot, M.; Rémond, D.; Combaret, L.; et al. A Dietary Supplementation with Leucine and Antioxidants Is Capable to Accelerate Muscle Mass Recovery after Immobilization in Adult Rats. PLoS ONE 2013, 8, e81495. [Google Scholar] [CrossRef] [PubMed]



| Cas-Cont | APP | Whey | SPI | EWP | |
|---|---|---|---|---|---|
| Protein, g/100 g | 86.2 | 96.9 | 87.0 | 87.9 | 80.4 |
| Fat, g/100 g | 1.0 | <0.1 | 0.2 | <0.1 | 0.7 |
| Amino acids, % | |||||
| Leucine | 8.4 | 7.5 | 12.9 | 6.6 | 7.4 |
| Valine | 5.8 | 4.5 | 5.1 | 3.8 | 5.8 |
| Isoleucine | 4.5 | 4.0 | 5.5 | 3.7 | 4.4 |
| Methionine | 2.5 | 2.9 | 2.4 | 1.1 | 3.1 |
| Threonine | 3.9 | 4.2 | 5.0 | 3.3 | 3.9 |
| Histidine | 2.7 | 2.2 | 2.0 | 2.3 | 2.0 |
| Phenylalanine | 4.6 | 3.6 | 3.5 | 4.5 | 6.2 |
| Tryptophan | 1.1 | 1.1 | 2.3 | 1.2 | 1.2 |
| Lysine | 7.1 | 8.6 | 10.8 | 5.3 | 5.9 |
| Arginine | 3.3 | 7.0 | 2.5 | 6.4 | 4.8 |
| Glycine | 1.7 | 4.2 | 1.7 | 3.5 | 3.0 |
| Glutamic acid | 20.0 | 14.1 | 16.9 | 15.9 | 11.6 |
| Alanine | 2.8 | 5.5 | 5.3 | 3.5 | 5.1 |
| Tyrosine | 5.0 | 3.3 | 3.6 | 3.2 | 3.3 |
| Aspartic acid | 6.4 | 9.6 | 11.8 | 9.9 | 9.0 |
| Serine | 5.2 | 4.0 | 3.9 | 4.4 | 6.7 |
| Cystine | 0.3 | 1.1 | 3.2 | 1.1 | 2.4 |
| Proline | 9.6 | 3.2 | 4.3 | 4.6 | 3.2 |
| Taurine | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 |
| Cas-Cont | APP | A- APP | Cas-Arg | 1/3 APP | 1/9 APP | Whey | SPI | EWP | |
|---|---|---|---|---|---|---|---|---|---|
| Ingredient, g/kg diet | - | - | - | - | - | ||||
| Casein | 200 | - | - | 200 | 133 | 178 | - | - | - |
| APP | - | 178 | - | - | 59 | 20 | - | - | - |
| A-APP | - | - | 172 | - | - | - | - | - | - |
| Whey | - | - | - | - | - | - | 198 | - | - |
| SPI | - | - | - | - | - | - | - | 196 | - |
| EWP | - | - | - | - | - | - | - | - | 215 |
| L-Cysteine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| L-Arginine | - | - | - | 4.4 | - | - | - | - | - |
| α-Cornstarch | 532 | 554 | 560 | 526.6 | 540 | 534 | 537 | 536 | 517 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| Lard | - | - | - | - | - | - | - | - | |
| AIN-93 mineral mixture | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| AIN-93 vitamin mixture | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Component, unit/kg diet | |||||||||
| Energy, kcal | 3876 | 3946 | 3970 | 3876 | 3887 | 3872 | 3882 | 3874 | 3812 |
| Protein, g | 172 | 172 | 172 | 172 | 172 | 172 | 172 | 172 | 172 |
| Fat, g | 72 | 70 | 70 | 70 | 71 | 72 | 70 | 70 | 72 |
| Final Body Weight | Body Weight Gain | Total Food Intake | |
|---|---|---|---|
| (g) | |||
| Exp. 1 | |||
| Cas-cont | 233 ± 3 | 57 ± 2 | 134 ± 3 |
| APP | 238 ± 4 | 61 ± 2 | 134 ± 3 |
| Cas-Arg | 235 ± 3 | 59 ± 1 | 137 ± 3 |
| Exp. 2 | |||
| Cas-cont | 233 ± 5 | 45 ± 2 | 117 ± 5 |
| APP | 239 ± 7 | 51 ± 3 * | 111 ± 4 |
| A-APP | 228 ± 3 | 40 ± 1 | 118 ± 2 |
| Exp. 3 | |||
| Cas-cont | 222 ± 3 | 60 ± 1 | 144 ± 3 |
| APP | 228 ± 3 | 65 ± 1 * | 142 ± 3 |
| A-APP | 216 ± 2 | 54 ± 1 * | 148 ± 3 |
| Exp. 4 | |||
| Cas-cont | 230 ± 2 | 64 ± 2 | 140 ± 2 |
| APP | 239 ± 2 * | 73 ± 2 ** | 143 ± 3 |
| 1/3 APP | 237 ± 3 | 71 ± 1 * | 139 ± 2 |
| 1/9 APP | 234 ± 3 | 68 ± 2 | 140 ± 3 |
| Exp. 5 | |||
| Cas-cont | 226 ± 3 | 54 ± 1 | 128 ± 3 |
| APP | 229 ± 3 | 57 ± 1 | 124 ± 3 |
| Whey | 220 ± 3 | 48 ± 2 * | 126 ± 3 |
| SPI | 220 ± 2 | 48 ± 1 * | 120 ± 2 |
| EWP | 223 ± 3 | 52 ± 2 | 124 ± 2 |
| Exp. 6 | |||
| Cas-cont 333 | 183 ± 3 | 15 ± 1 | 36 ± 1 |
| APP 333 | 184 ± 2 | 15 ± 1 | 35 ± 1 |
| Saline | 185 ± 2 | 15 ± 1 | 36 ± 1 |
| Cas-Cont | APP | Cas-Arg | |
|---|---|---|---|
| Val | 247 ± 12 b | 169 ± 8 a | 226 ± 8 b |
| Leu | 130 ± 10 | 111 ± 8 | 116 ± 6 |
| Ile | 92 ± 5 | 89 ± 5 | 83 ± 3 |
| Arg | 90 ± 4 a | 150 ± 11 b | 117 ± 11 a |
| Gly | 121 ± 5 a | 251 ± 9 b | 116 ± 4 b |
| Pro | 446 ± 21 b | 160 ± 10 a | 427 ± 20 b |
| Lys | 389 ± 15 ab | 468 ± 33 b | 378 ± 22 a |
| Asp | 26 ± 3 | 24 ± 3 | 25 ± 3 |
| Thr | 472 ± 38 | 491 ± 27 | 447 ± 44 |
| Ser | 227 ± 10 | 214 ± 10 | 200 ± 12 |
| Asn | 71 ± 2 b | 58 ± 3 a | 63 ± 2 ab |
| Glu | 129 ± 8 | 126 ± 9 | 121 ± 8 |
| Gln | 702 ± 25 b | 586 ± 17 a | 663 ± 22 ab |
| Ala | 473 ± 23 | 449 ± 42 | 412 ± 19 |
| Cys | 33 ± 2 | 37 ± 3 | 36 ± 3 |
| Met | 75 ± 3 ab | 80 ± 4 b | 68 ± 3 a |
| Tyr | 89 ± 5 c | 53 ± 3 a | 72 ± 3 b |
| Phe | 52 ± 3 | 47 ± 2 | 47 ± 2 |
| Trp | 109 ± 3 b | 101 ± 2 a | 105 ± 2 ab |
| His | 52 ± 2 c | 39 ± 2 a | 45 ± 2 b |
| Cas-Cont | APP | Cas-Arg | |
|---|---|---|---|
| Val | 225 ± 7 c | 147 ± 5 a | 204 ± 7 b |
| Leu | 110 ± 6 b | 85 ± 5 a | 104 ± 6 b |
| Ile | 87 ± 3 | 76 ± 4 | 83 ± 3 |
| Arg | 65 ± 8 a | 196 ± 21 c | 139 ± 10 b |
| Gly | 1981 ± 79 a | 3294 ± 96 b | 1840 ± 87 a |
| Pro | 1068 ± 52 b | 269 ± 9 a | 971 ± 30 b |
| Lys | 600 ± 52 a | 852 ± 95 b | 617 ± 46 a |
| Asp | 192 ± 16 | 150 ± 13 | 161 ± 17 |
| Thr | 1228 ± 89 | 1092 ± 43 | 1141 ± 84 |
| Ser | 1041 ± 45 b | 770 ± 27 a | 924 ± 47 b |
| Asn | 304 ± 14 c | 173 ± 4 a | 257 ± 6 b |
| Glu | 907 ± 55 | 755 ± 38 | 903 ± 61 |
| Gln | 5787 ± 216 b | 4217 ± 128 a | 5270 ± 137 a |
| Ala | 3013 ± 87 c | 2210 ± 65 a | 2566 ± 75 b |
| Cys | n.d. | n.d. | n.d. |
| Met | 86 ± 2 | 87 ± 2 | 81 ± 3 |
| Tyr | 102 ± 7 c | 55 ± 3 a | 83 ± 4 b |
| Phe | 49 ± 2 b | 41 ± 1 a | 49 ± 3 b |
| Trp | 23 ± 3 | 22 ± 4 | 21 ± 3 |
| His | 213 ± 8 c | 124 ± 6 a | 180 ± 2 b |
| Tau | 7799 ± 217 | 7910 ± 258 | 7481 ± 251 |
| Cas-Cont | APP | A-APP | |
|---|---|---|---|
| Val | 303 ± 24 b | 176 ± 6 a | 133 ± 7 a |
| Leu | 177 ± 18 b | 124 ± 5 a | 103 ± 9 a |
| Ile | 113 ± 11 b | 95 ± 3 b | 61 ± 5 a |
| Arg | 113 ± 6 a | 176 ± 13 b | 196 ± 18 b |
| Gly | 122 ± 5 a | 275 ± 8 b | 468 ± 21 c |
| Pro | 560 ± 26 c | 169 ± 6 a | 282 ± 26 b |
| Lys | 462 ± 16 a | 596 ± 50 a | 844 ± 71 b |
| Asp | 31 ± 5 | 34 ± 2 | 27 ± 4 |
| Thr | 413 ± 13 a | 387 ± 21 a | 970 ± 113 b |
| Ser | 228 ± 13 a | 226 ± 5 a | 398 ± 20 b |
| Asn | 90 ± 5 c | 73 ± 2 b | 33 ± 2 a |
| Glu | 141 ± 15 | 157 ± 7 | 143 ± 11 |
| Gln | 626 ± 27 b | 526 ± 23 a | 455 ± 14 a |
| Ala | 540 ± 37 a | 535 ± 40 a | 798 ± 43 b |
| Cys | 46 ± 2 a | 40 ± 3 a | 54 ± 2 b |
| Met | 86 ± 4 | 85 ± 1 | 80 ± 3 |
| Tyr | 113 ± 12 b | 62 ± 2 a | 73 ± 7 a |
| Phe | 63 ± 4 b | 47 ± 1 a | 44 ± 2 a |
| Trp | 119 ± 7 b | 100 ± 5 ab | 85 ± 7 a |
| His | 55 ± 3 b | 43 ± 1 a | 61 ± 5 b |
| Cas-cont | APP | 1/3 APP | 1/9 APP | |
|---|---|---|---|---|
| Val | 306 ± 9 a | 191 ± 14 c | 226 ± 5 bc | 245 ± 8 b |
| Leu | 189 ± 9 a | 131 ± 10 b | 137 ± 4 b | 143 ± 7 b |
| Ile | 127 ± 5 a | 102 ± 7 b | 99 ± 2 b | 98 ± 4 b |
| Arg | 117 ± 4 b | 179 ± 13 a | 127 ± 4 b | 103 ± 4 b |
| Gly | 135 ± 2 c | 245 ± 15 a | 168 ± 5 b | 137 ± 5 bc |
| Pro | 556 ± 16 a | 163 ± 12 d | 331 ± 10 c | 412 ± 15 b |
| Lys | 527 ± 28 ab | 582 ± 51 a | 482 ± 26 ab | 447 ± 22 b |
| Asp | 30 ± 3 | 29 ± 3 | 30 ± 3 | 29 ± 2 |
| Thr | 508 ± 35 | 513 ± 44 | 474 ± 28 | 466 ± 31 |
| Ser | 243 ± 5 | 215 ± 14 | 215 ± 5 | 211 ± 9 |
| Asn | 96 ± 3 a | 68 ± 5 b | 74 ± 2 b | 75 ± 2 b |
| Glu | 171 ± 11 | 158 ± 17 | 156 ± 8 | 151 ± 9 |
| Gln | 666 ± 21 a | 469 ± 35 c | 571 ± 17 b | 588 ± 9 ab |
| Ala | 567 ± 14 a | 481 ± 37 b | 431 ± 8 b | 436 ± 13 b |
| Cys | 38 ± 1 | 38 ± 2 | 31 ± 4 | 34 ± 1 |
| Met | 80 ± 2 ab | 86 ± 6 a | 75 ± 2 ab | 72 ± 2 b |
| Tyr | 104 ± 6 a | 65 ± 5 b | 81 ± 3 b | 81 ± 7 b |
| Phe | 65 ± 2 a | 50 ± 3 b | 55 ± 2 b | 54 ± 2 b |
| Trp | 128 ± 4 | 114 ± 9 | 115 ± 5 | 112 ± 4 |
| His | 69 ± 4 a | 41 ± 3 c | 54 ± 1 b | 55 ± 1 b |
| EAA | 2000 ± 61 a | 1810 ± 133 ab | 1717 ± 47 ab | 1691 ± 59 b |
| BCAA | 622 ± 22 a | 424 ± 31 b | 462 ± 11 b | 486 ± 19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, K.; Fujitani, M.; Mizushige, T.; Hayamizu, K.; Hara, Y.; Sawai, M.; Utsunomiya, S.; Uehigashi, R.; Okada, S.; Kishida, T. Dietary Alaska Pollack Protein Induces Acute Skeletal Muscle Hypertrophy in Rats, Regardless of Specific Amino Acid and Amino Acid Balance of Diet. Nutraceuticals 2023, 3, 513-528. https://doi.org/10.3390/nutraceuticals3040037
Uchida K, Fujitani M, Mizushige T, Hayamizu K, Hara Y, Sawai M, Utsunomiya S, Uehigashi R, Okada S, Kishida T. Dietary Alaska Pollack Protein Induces Acute Skeletal Muscle Hypertrophy in Rats, Regardless of Specific Amino Acid and Amino Acid Balance of Diet. Nutraceuticals. 2023; 3(4):513-528. https://doi.org/10.3390/nutraceuticals3040037
Chicago/Turabian StyleUchida, Kenji, Mina Fujitani, Takafumi Mizushige, Kohsuke Hayamizu, Yuma Hara, Mariko Sawai, Sachi Utsunomiya, Ryota Uehigashi, Shinji Okada, and Taro Kishida. 2023. "Dietary Alaska Pollack Protein Induces Acute Skeletal Muscle Hypertrophy in Rats, Regardless of Specific Amino Acid and Amino Acid Balance of Diet" Nutraceuticals 3, no. 4: 513-528. https://doi.org/10.3390/nutraceuticals3040037