The Marine Factor 3,5-Dihydroxy-4-methoxybenzyl Alcohol Represses Adipogenesis in Mouse 3T3-L1 Adipocytes In Vitro: Regulating Diverse Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. 3,5-Dihydroxy-4-methoxybenzyl Alcohol
2.3. 3T3-L1 Preadipocytes
2.4. Assay of Cell Growth and Death
2.5. Differentiation of 3T3-L1 Preadipocytes and Assay of Lipid Droplets
2.6. Assay of Adipogenesis in 3T3-L1 Adipocytes
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Effects of DHMBA on the Proliferation, Death or Growth of 3T3-L1 Preadipocytes
3.2. Effects of DHMBA on Cell Growth with Differentiation Process of 3T3-L1 Preadipocytes
3.3. DHMBA Inhibits Lipid Accumulation in 3T3-L1 Adipocytes
3.4. DHMBA Represses Adipogenesis in 3T3-L1 Adipocytes
3.5. Effects of DHMBA on Adipogenesis in 3T3-L1 Adipocytes Cultured with the Inhibitor of Intracellular Signaling Pathway
3.6. Effects of DHMBA on the Levels of Proteins Related to Signaling Process of Adipogenesis
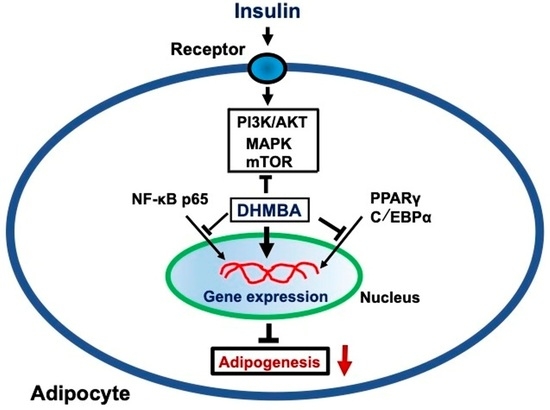
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wickelgren, I. Obesity: How Big a Problem? Science 1998, 280, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.I.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Morison, R.I.; Farmer, S.R. Insight into the transcriptional control of adipocyte differentiation. J. Cell Biochem. 1999, 75, 59–67. [Google Scholar] [CrossRef]
- Laudes, M. Role of WNT signaling in the determination of human mesenchymal stem cells into preadipocytes. J. Mol. Endocrinol. 2001, 46, R65–R72. [Google Scholar]
- Green, H.; Kehinde, O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 1976, 7, 105–113. [Google Scholar] [CrossRef]
- Bernlohr, D.; Bolanowski, M.; Kelly, T.J.; Lane, M.D. Evidence for an increase in transcription of specific mRNA during differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 1985, 260, 5563–5567. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of inflammatory cytokines, growth factors and adipokines in adipogenesis and insulin resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and characterization of a phenolic antioxidant from the pacific oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef]
- Fuda, H.; Watanabe, M.; Hui, S.-P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Gigas, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef]
- Joko, S.; Watanabe, M.; Fuda, H.; Takeda, S.; Furukawa, T.; Hui, S.-P.; Shrestha, R.; Chiba, H. Comparison of chemical structures and cytoprotection abilities between direct and indirect antioxidants. J. Funct. Foods 2017, 35, 245–255. [Google Scholar] [CrossRef]
- Tamano, H.; Shakushi, Y.; Watanabe, M.; Ohashi, K.; Uematsu, C.; Otsubo, T.; Ikeda, K.; Takeda, A. Preventive effect of 3,5-dihydroxy-4-methoxybenzyl alcohol (DHMBA) and zinc, components of the pacific oyster Crassostrea gigas, on glutamatergic neuron activity in the hippocampus. J. Agric. Food Chem. 2019, 67, 12844–12853. [Google Scholar] [CrossRef]
- Fukai, M.; Nakayabu, T.; Ohtani, S.; Shibata, K.; Shimada, S.; Sakamoto, S.; Fuda, H.; Furukawa, T.; Watanabe, M.; Hui, S.-P.; et al. The Phenolic Antioxidant 3,5-dihydroxy-4-methoxybenzyl Alcohol (DHMBA) prevents enterocyte cell death under oxygen-dissolving cold conditions through polyphyletic antioxidant actions. J. Clin. Med. 2021, 10, 1972. [Google Scholar] [CrossRef]
- Okabe, H.; Hui, S.-P.; Fuda, H.; Furukawa, T.; Takeda, S.; Shrestha, R.; Miura, Y.; Watanabe, M.; Chiba, H. Mass Spectrometric quantification of amphipathic, polyphenolic antioxidant of the pacific oyster (Crassostrea gigas). Anal. Sci. 2015, 31, 1341–1344. [Google Scholar] [CrossRef] [Green Version]
- Villuendas-Rey, Y.; Alvarer-Idaboy, J.R.; Galano, A. Assessing the protective activity of a recently discovered phenolic compound against oxidative stress using computational chemistry. J. Chem. Int. Model. 2015, 55, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The marine factor 3,5-dihydroxy-4-methoxybenzyl alcohol suppresses growth, migration, and invasion and stimulates death of metastatic human prostate cancer cells: Targeting diverse signaling processes. Anticancer Drug 2022, 33, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Yamaguchi, M.; Kohno, S.; Takahashi, C.; Watanabe, R.; Hatori, K.; Hikita, K.; Kaneda, N. Regucalcin enhances adipocyte differentiation and attenuates inflammation in 3T3-L1 cells. FEBS Open Bio. 2020, 10, 1967–1984. [Google Scholar] [CrossRef]
- Misawa, H.; Inagaki, S.; Yamaguchi, M. Suppression of cell proliferation and deoxyribonucleic acid synthesis in cloned rat hepatoma H4-II-E cells overexpressing regucalcin. J. Cell Biochem. 2002, 84, 143–149. [Google Scholar] [CrossRef]
- Izumi, T.; Yamaguchi, M. Overexpression of regucalcin suppresses cell death in cloned rat hepatoma H4-II-E cells induced by tumor necrosis factor-α or thapsigargin. J. Cell Biochem. 2004, 92, 296–306. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N.; Baile, C.A.; Murata, T. Exogenous regucalcin suppresses osteoblastogenesis and stimulates adipogenesis in mouse bone marrow culture. Integ. Biol. 2012, 4, 1215–1222. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Baile, C.A.; Zhu, S.; Shoji, M. Bioactive flavonoid p-hydroxycinnamic acid stimulates osteoblastogenesis and suppresses adipogenesis in bone marrow culture. Cell Tissue Res. 2013, 354, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Otley, M.O.C.; Sinal, C.J. Adipocyte-cancer cell interactions in the bone microenvironment. Front. Endocrinol. 2022, 13, 903926. [Google Scholar] [CrossRef] [PubMed]
- Hemati, N.; Ross, S.E.; Erickson, R.L.; Manson, M.M.; Walker, R.A.; Gescher, A. Signaling pathways through which insulin regulates CCAAT/enhance binding protein alpha (C/EBPalpha) phosphorylation and gene expression in 3T3-L1 adipocytes: Correlation with GLUT4 gene expression. J. Biol. Chem. 1997, 272, 25913–25919. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanism and therapeutic interventions. Signal. Transd. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Phytochemical genistein in the regulation of vascular function: New insight. Curr. Med. Chem. 2007, 14, 2581–2589. [Google Scholar] [CrossRef]
- Peleck, S.L.; Charest, D.L.; Mordret, G.P.; Siow, Y.L.; Palaty, C.; Campbell, D.; Chaslton, L.; Samiei, M.; Sanghera, J.S. Networking with mitogen-activated protein kinases. Mol. Cell Biochem. 1993, 127, 157–169. [Google Scholar] [CrossRef]
- Serrano-Nascimento, C.; da Silva Teixeira, S.; Nicola, J.P.; Nachbar, R.T.; Masini-Repiso, A.M.; Nunes, M.T. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signaling pathway. Endocrinology 2014, 155, 1145–1156. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.A.; Schattner, E.J.; Cesarman, E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 2000, 96, 2537–2542. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, J.; Xiong, W.; Chen, S.; Fan, L.; Li, Y. Notch3 promotes 3T3-L1 pre adipocytes differentiation by up-regulating the expression of LARS to activate the mTOR pathway. J. Cell Mol. Med. 2020, 24, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Grancieri, M.; Martino, H.S.D.; Gonzalex de Mejia, E. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB pathways in 3T3-L1 adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The Marine Factor 3,5-Dihydroxy-4-methoxybenzyl Alcohol Represses Adipogenesis in Mouse 3T3-L1 Adipocytes In Vitro: Regulating Diverse Signaling Pathways. Nutraceuticals 2023, 3, 366-379. https://doi.org/10.3390/nutraceuticals3030028
Yamaguchi M, Yoshiike K, Watanabe H, Watanabe M. The Marine Factor 3,5-Dihydroxy-4-methoxybenzyl Alcohol Represses Adipogenesis in Mouse 3T3-L1 Adipocytes In Vitro: Regulating Diverse Signaling Pathways. Nutraceuticals. 2023; 3(3):366-379. https://doi.org/10.3390/nutraceuticals3030028
Chicago/Turabian StyleYamaguchi, Masayoshi, Kenji Yoshiike, Hideaki Watanabe, and Mitsugu Watanabe. 2023. "The Marine Factor 3,5-Dihydroxy-4-methoxybenzyl Alcohol Represses Adipogenesis in Mouse 3T3-L1 Adipocytes In Vitro: Regulating Diverse Signaling Pathways" Nutraceuticals 3, no. 3: 366-379. https://doi.org/10.3390/nutraceuticals3030028