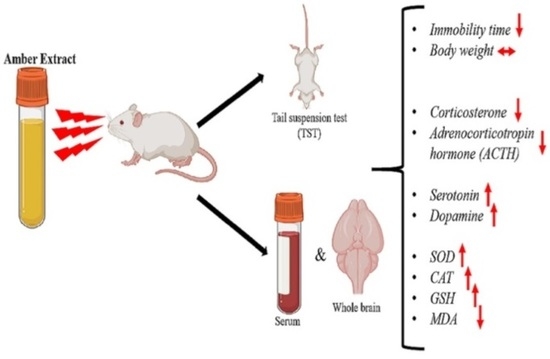
Behavioral and Biochemical Evaluation of Anti-Depressive and Oxidative Stress-Ameliorating Effects of Amber Extract in Adult Male ICR Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amber Extract and Other Reagents
2.2. Animals
2.3. Sample Collection
2.4. Tissue Homogenization and Protein Quantification
2.5. Tail Suspension Test (TST)
2.6. Determination of the Levels of Hormones and Neurotransmitters
2.7. Determination of Oxidative Stress Parameters
2.8. Statistical Analysis
3. Results
3.1. Amber Treatment Did Not Affect the Body Weight of Mice
3.2. Amber Supplementation Showed Antidepressant Effect in Tail-Suspended Mice
3.3. Amber Positively Regulated Hormones in the HPA Axis System
3.4. Amber Treatment Positively Controlled Antidepressant Hormones
3.5. Amber Ameliorated Oxidative Stress in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative stress and major depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef] [PubMed]
- Sannino, G.; Pasqualini, L.; Ricciardelli, E.; Montilla, P.; Soverchia, L.; Ruggeri, B.; Falcinelli, S.; Renzi, A.; Ludka, C.; Kirchner, T.; et al. Acute stress enhances the expression of neuroprotection- and neurogenesis-associated genes in the hippocampus of a mouse restraint model. Oncotarget 2016, 7, 8455–8465. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Michel, T.M.; Pülschen, D.; Thome, J. The role of oxidative stress in depressive disorders. Curr. Pharm. Des. 2012, 18, 5890–5899. [Google Scholar] [CrossRef]
- Sulakhiya, K.; Patel, V.K.; Saxena, R.; Dashore, J.; Srivastava, A.K.; Rathore, M. Effect of Beta vulgaris Linn. leaves extract on anxiety–and depressive-like behavior and oxidative stress in mice after acute restraint stress. Pharmacogn. Res. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: A double-blind, randomized, placebo-controlled clinical study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Muruganandam, A.V. Adaptogenic activity of Withania somnifera: An experimental study using a rat model of chronic stress. Pharmacol. Biochem. Behav. 2003, 75, 547–555. [Google Scholar] [CrossRef]
- Rai, D.; Bhatia, G.; Sen, T.; Palit, G. Anti-stress effects of Ginkgo biloba and Panax ginseng: A comparative study. J. Pharmacol. Sci. 2003, 93, 458–464. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Li, Q.S.; Xie, S.X.; Mao, J.J. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J. Altern. Complement. Med. 2020, 26, 813–819. [Google Scholar] [CrossRef]
- Somuah-Asante, S.; Sakamoto, K. Stress buffering and longevity effects of amber extract on Caenorhabditis elegans (C. elegans). Molecules 2022, 27, 3858. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, S.; Haeiwa, H.; Takeda, R.; Okazaki, K.; Sekita, M.; Yamamoto, T.; Yamano, M.; Sakamoto, K. Role of amber extract in protecting SHSY5Y cells against amyloid β1-42-induced neurotoxicity. Biomed. Pharmacother. 2021, 141, 111804. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, S.; Takeda, R.; Okazaki, K.; Sekita, M.; Sakamoto, K. Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 141, 111854. [Google Scholar] [CrossRef] [PubMed]
- Sogo, E.; Zhou, S.; Haeiwa, H.; Takeda, R.; Okazaki, K.; Sekita, M.; Yamamoto, T.; Yamano, M.; Sakamoto, K. Amber extract reduces lipid content in mature 3T3-L1 adipocytes by activating the lipolysis pathway. Molecules 2021, 26, 4630. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, W.H.; Al-Saadi, S.A.A.M.; Burghal, A.A. Antibacterial activity and GC-MS analysis of baltic amber against pathogenic bacteria. Int. J. Adv. Sci. Technol. 2020, 29, 611–618. [Google Scholar]
- Villareal, M.O.; Ikeya, A.; Sasaki, K.; Arfa, A.B.; Neffati, M.; Isoda, H. Anti-stress and neuronal cell differentiation induction effects of Rosmarinus officinalis L. essential oil. BMC Complement. Altern. Med. 2017, 17, 549. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Zhu, W.-L.; Shi, H.-S.; Wei, Y.-M.; Wang, S.-J.; Sun, C.-Y.; Ding, Z.-B.; Lu, L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012, 65, 74–80. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Garrone, G.; Dick, P. Monoamine oxidase inhibitors in the treatment of depressive states. Psychiatr. Neurol. 1960, 140, 107–114. [Google Scholar] [CrossRef]
- Haenisch, B.; Bönisch, H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 2011, 129, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, M.; Linda, F.K.; Ramesh, S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Ah Kang, K.; Zhang, R.; Piao, M.J.; Jo, S.H.; Kim, J.S.; Kang, S.S.; Lee, J.S.; Park, D.H.; Hyun, J.W. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Pharmacol. 2010, 29, 12–18. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somuah-Asante, S.; Othman, M.B.; Takeda, R.; Okazaki, K.; Sekita, M.; Sakamoto, K. Behavioral and Biochemical Evaluation of Anti-Depressive and Oxidative Stress-Ameliorating Effects of Amber Extract in Adult Male ICR Mice. Nutraceuticals 2023, 3, 222-230. https://doi.org/10.3390/nutraceuticals3020017
Somuah-Asante S, Othman MB, Takeda R, Okazaki K, Sekita M, Sakamoto K. Behavioral and Biochemical Evaluation of Anti-Depressive and Oxidative Stress-Ameliorating Effects of Amber Extract in Adult Male ICR Mice. Nutraceuticals. 2023; 3(2):222-230. https://doi.org/10.3390/nutraceuticals3020017
Chicago/Turabian StyleSomuah-Asante, Sandra, Mahmoud Ben Othman, Reiko Takeda, Kazuma Okazaki, Marie Sekita, and Kazuichi Sakamoto. 2023. "Behavioral and Biochemical Evaluation of Anti-Depressive and Oxidative Stress-Ameliorating Effects of Amber Extract in Adult Male ICR Mice" Nutraceuticals 3, no. 2: 222-230. https://doi.org/10.3390/nutraceuticals3020017