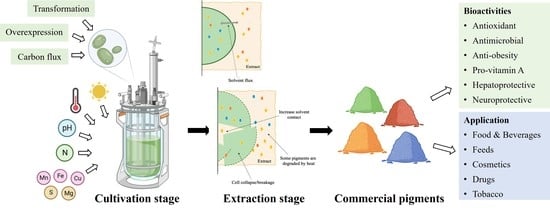
Microalgae-Derived Pigments for the Food Industry
Abstract
:1. Introduction
2. Chemistry and Biochemistry of Microalgal Pigments
3. Potential Application of Microalgal Pigments and Target Foods
3.1. Chlorophylls
3.2. Carotenoids
3.3. Phycobiliproteins
4. Factors Affecting the Microalgal Pigment Production
4.1. Light
4.2. Temperature
4.3. Culture Media
4.3.1. Nitrogen
4.3.2. PH and Salinity
4.3.3. Micronutrients
5. Metabolic and Genomic Design for Pigment Production
6. Downstream Processing for the Stability of Microalgal Pigments
6.1. Pigment Stability by Extraction Technology
6.1.1. Classic Methods
6.1.2. Pressurized Systems
6.1.3. Wave-Energy Treatment
6.1.4. Enzymatic Extraction
6.1.5. Pulsed Electric Field (PEF)
6.1.6. Novel Methodologies
6.2. Pigment Stability in Food Processing and Improving Strategies
7. Economic Analysis of Microalgal Pigments in Foods
8. Future Perspective and Conclusive Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Ntan, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L. The Next Big Superfood Could Be Green and Slimy. Available online: https://www.businessinsider.com/algae-is-the-superfood-of-the-future-2014-6 (accessed on 22 January 2023).
- Silva, S.C.; Ferreira, I.C.; Dias, M.M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Lopez, M.J.; Hall, C.A. Physiology, Osmosis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Di Dong, C. Advances in micro-and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Ferreira, V.D.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 8. [Google Scholar]
- Juric, S.; Juric, M.; Krol-Kilinska, Z.; Vlahovicek-Kahlina, K.; Vincekovic, M.; Dragovic-Uzelac, V.; Donsi, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.; Spijkerman, A.; Van der Schouw, Y. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef]
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 2018, 135, 54–61. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chuyen, H.V.; Eun, J.-B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Shakeri, A.; Soheili, V.; Karimi, M.; Hosseininia, S.A.; Fazly Bazzaz, B.S. Biological activities of three natural plant pigments and their health benefits. J. Food Meas. Charact. 2018, 12, 356–361. [Google Scholar] [CrossRef]
- Du, S.-Y.; Zhang, Y.-L.; Bai, R.-X.; Ai, Z.-L.; Xie, B.-S.; Yang, H.-Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785. [Google Scholar]
- Huang, Y.-M.; Dou, H.-L.; Huang, F.-F.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-X.; Liu, T.; Dai, X.-L.; Zheng, Q.-S.; Hui, B.-D.; Jiang, Z.-F. Treatment with lutein provides neuroprotection in mice subjected to transient cerebral ischemia. J. Asian Nat. Prod. Res. 2014, 16, 1084–1093. [Google Scholar] [CrossRef]
- Hosokawa, M.; Wanezaki, S.; Miyauchi, K.; Kurihara, H.; Kohno, H.; Kawabata, J.; Odashima, S.; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res. 1999, 5, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Duppeti, H.; Chakraborty, S.; Das, B.S.; Mallick, N.; Kotamreddy, J. Rapid assessment of algal biomass and pigment contents using diffuse reflectance spectroscopy and chemometrics. Algal Res. 2017, 27, 274–285. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1. 40. under optimized culture conditions. J. Basic Microbiol. 2022, 62, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, X.; Ren, Y.; Zhang, H.; Mao, X.; Lao, Y.; Wang, X.; Chen, F. Boost carbon availability and value in algal cell for economic deployment of biomass. Bioresour. Technol. 2020, 300, 122640. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Colozzi-Filho, A.; Guedes, C.; Lima, F.; Machineski, G.; Matos, M. Principais produtos da biomassa algal e suas aplicações biotecnológicas. In Microalgas De Águas Cont. Potencialidades E Desafios Do Cultiv; Londrina: Iapar, Brazil, 2014; pp. 265–343. [Google Scholar]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, H.; Wu, T.; Fu, Y.L.; He, Y.J.; Mao, X.M.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 2021, 533, 736074. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-adapted microalgae for fucoxanthin production: Effect of incremental increase in salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Bermejo, R.; Acién, F.G.; Ibáñez, M.J.; Fernández, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.; Fuentes, J.L.; Mendiola, J.; Cerezal-Mezquita, P.; Morales, J.; Vílchez, C.; Ibáñez, E. Bioprospecting of cyanobacterium in Chilean coastal desert, Geitlerinema sp. molecular identification and pressurized liquid extraction of bioactive compounds. Food Bioprod. Process. 2021, 128, 227–239. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Alonso-Gonzalez, A.; Orive, E.; David, H.; Garcia-Etxebarria, K.; Garrido, J.L.; Laza-Martinez, A.; Seoane, S. Scaly green flagellates from Spanish Atlantic coastal waters: Molecular, ultrastructural and pigment analyses. Bot. Mar. 2014, 57, 379–402. [Google Scholar] [CrossRef] [Green Version]
- Fithriani, D.; Ambarwaty, D.; Nurhayati. Identification of Bioactive Compounds from Nannochloropsis sp. 4th ed. In Proceedings of the EMBRIO International Symposium (EIS)/7th International Symposium of East-Asia-Fisheries-and-Technologists-Association (EAFTA), Bogor, Indonesia, 5–6 August 2019. [Google Scholar]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of Marine Phytoplankton Produce Bioactive Pigments and Lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Molecular structure, stability and cytotoxicity of natural green colorants produced from Centella asiatica L. leaves treated by steaming and metal complexations. Food Chem. 2017, 232, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Innovative microalgae pigments as functional ingredients in nutrition. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–243. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biot 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Marawaha, R.K.; Bansal, D.; Kaur, S.; Trehan, A. Wheat grass juice reduces transfusion requirement in patients with thalassemia major: A pilot study. Indian Pediatr. 2004, 41, 716–720. [Google Scholar] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 867–901. [Google Scholar]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of Microalgae Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2017, 17, 1140–1172. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Chiu, H.F.; Liao, J.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Anti-proliferative, anti-inflammatory and pro-apoptotic effects of Dunaliella salina on human KB oral carcinoma cells. J. Food Biochem. 2017, 41, e12349. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.D.; Perez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of Microalgae Carotenoids for Industrial Application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Ohi, Y.; Namiki, T.; Katatae, M.; Tsukahara, H.; Kitamura, A. Effects of the addition of the natural carotenoid astaxanthin from microalgae Haematococcus pluvialis on the physical properties of bread. Nippon Shokuhin Kagaku Kogaku Kaishi = J. Jpn. Soc. Food Sci. Technol. 2009, 56, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Sun, H.; Yang, S.; Zhao, W.; Kong, Q.; Zhu, C.; Fu, X.; Zhang, F.; Liu, Z.; Zhan, Y.; Mou, H.; et al. Fucoxanthin from marine microalgae: A promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.-W.; Chen, F. Fucoxanthinol from the diatom Nitzschia laevis ameliorates neuroinflammatory responses in lipopolysaccharide-stimulated BV-2 microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochem. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.; Vatsala, T. Pigment production in Spirulina fussiformis in different photophysical conditions. Biomol. Eng. 2007, 24, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, U.; Pathak, N. Effect of different conditions on the production of chlorophyll by Spirulina platensis. J. Algal Biomass Utln 2010, 1, 89–99. [Google Scholar]
- Pisal, D.S.; Lele, S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Imamoglu, E.; Dalay, M.C.; Sukan, F.V. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotech. 2009, 26, 199–204. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.-H.; Xie, Y.; Chen, J. Strategies related to light quality and temperature to improve lutein production of marine microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Kim, Z.-H.; Kim, S.-H.; Lee, H.-S.; Lee, C.-G. Enhanced production of astaxanthin by flashing light using Haematococcus pluvialis. Enzym. Microb. Technol. 2006, 39, 414–419. [Google Scholar] [CrossRef]
- Fatma, T. Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar]
- Chen, W.-C.; Hsu, Y.-C.; Chang, J.-S.; Ho, S.-H.; Wang, L.-F.; Wei, Y.-H. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wu, Y.; Zhang, L. Effect of light quality on growth, ultrastructure, pigments, and membrane lipids of Pyropia haitanensis. J. Appl. Phycol. 2020, 32, 4189–4197. [Google Scholar] [CrossRef]
- Wu, H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res. Int. 2016, 2016, 7383918. [Google Scholar] [CrossRef] [Green Version]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Domınguez-Bocanegra, A.; Legarreta, I.G.; Jeronimo, F.M.; Campocosio, A.T. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2004, 92, 209–214. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Kang, C.D.; An, J.Y.; Park, T.H.; Sim, S.J. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Flores, É.M.; Barin, J.S.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014, 65, 144–148. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodrıguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.N.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, F.; Chen, G.; Zhang, X.; Liu, L.; Zhang, H. Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci. China Ser. Life Sci. 2008, 51, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum-a prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Lv, J.M.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour. Technol. 2010, 101, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Mojaat, M.; Pruvost, J.; Foucault, A.; Legrand, J. Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem. Eng. J. 2008, 39, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Esakkimuthu, S.; Krishnamurthy, V.; Govindarajan, R.; Swaminathan, K. Augmentation and starvation of calcium, magnesium, phosphate on lipid production of Scenedesmus obliquus. Biomass Bioenergy 2016, 88, 126–134. [Google Scholar] [CrossRef]
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef]
- Jerez, C.G.; Malapascua, J.R.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Effect of nutrient starvation under high irradiance on lipid and starch accumulation in Chlorella fusca (Chlorophyta). Mar. Biotechnol. 2016, 18, 24–36. [Google Scholar] [CrossRef]
- Yamazaki, T.; Konosu, E.; Takeshita, T.; Hirata, A.; Ota, S.; Kazama, Y.; Abe, T.; Kawano, S. Independent regulation of the lipid and starch synthesis pathways by sulfate metabolites in the green microalga Parachlorella kessleri under sulfur starvation conditions. Algal Res. 2018, 36, 37–47. [Google Scholar] [CrossRef]
- Scarsini, M.; Marchand, J.; Schoefs, B. Carotenoid Overproduction in Microalgae: Biochemical and Genetic Engineering. In Pigments from Microalgae Handbook; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolphanderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B.; et al. Chloroplast Transformation in Chlamydomonas with High-Velocity Microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef]
- Purton, S.; Szaub, J.B.; Wannathong, T.; Young, R.; Economou, C.K. Genetic engineering of algal chloroplasts: Progress and prospects. Russ. J. Plant Physiol. 2013, 60, 491–499. [Google Scholar] [CrossRef]
- Kilian, O.; Benemann, C.S.E.; Niyogi, K.K.; Vick, B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef] [Green Version]
- Radakovits, R.; Eduafo, P.M.; Posewitz, M.C. Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab. Eng. 2011, 13, 89–95. [Google Scholar] [CrossRef]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biot. 2016, 153, 1–35. [Google Scholar]
- Yang, B.; Liu, J.; Liu, B.; Sun, P.P.; Ma, X.N.; Jiang, Y.; Wei, D.; Chen, F. Development of a stable genetic system for Chlorella vulgaris-A promising green alga for CO2 biomitigation. Algal Res. 2015, 12, 134–141. [Google Scholar] [CrossRef]
- Bock, R. Engineering Plastid Genomes: Methods, Tools, and Applications in Basic Research and Biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar] [CrossRef] [Green Version]
- Couso, I.; Vila, M.; Rodriguez, H.; Vargas, M.A.; Leon, R. Overexpression of an Exogenous Phytoene Synthase Gene in the Unicellular Alga Chlamydomonas reinhardtii Leads to an Increase in the Content of Carotenoids. Biotechnol. Prog. 2011, 27, 54–60. [Google Scholar] [CrossRef]
- Kadono, T.; Kira, N.; Suzuki, K.; Iwata, O.; Ohama, T.; Okada, S.; Nishimura, T.; Akakabe, M.; Tsuda, M.; Adachi, M. Effect of an Introduced Phytoene Synthase Gene Expression on Carotenoid Biosynthesis in the Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2015, 13, 5334–5357. [Google Scholar] [CrossRef] [Green Version]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henriquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res.-Biomass Biofuels Bioprod. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Lin, B.; Cui, Y.L.; Yan, M.Y.; Wang, Y.C.; Gao, Z.Q.; Meng, C.X.; Qin, S. Construction of astaxanthin metabolic pathway in the green microalga Dunaliella viridis. Algal Res.-Biomass Biofuels Bioprod. 2019, 44, 101697. [Google Scholar] [CrossRef]
- Tran, Q.G.; Cho, K.; Kim, U.; Yun, J.H.; Cho, D.H.; Heo, J.; Park, S.B.; Kim, J.W.; Lee, Y.J.; Ramanan, R.; et al. Enhancement of beta-carotene production by regulating the autophagy-carotenoid biosynthesis seesaw in Chlamydomonas reinhardtii. Bioresour. Technol. 2019, 292, 121937. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, T.; Chen, S.H.Y.; Ren, Y.; Yang, S.; Huang, J.; Mou, H.; Chen, F. Powerful tools for productivity improvements in microalgal production. Renew. Sustain. Energy Rev. 2021, 152, 111609. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Fan, Y.; Lu, X.; Zhao, W.; Chen, F. Systematic metabolic tools reveal underlying mechanism of product biosynthesis in Chromochloris zofingiensis. Bioresour. Technol. 2021, 337, 125406. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.-W.; Chen, F.; Liu, B. A Hetero-Photoautotrophic Two-Stage Cultivation Process for Production of Fucoxanthin by the Marine Diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2021, 347, 126401. [Google Scholar] [CrossRef]
- Sun, H.; Kong, Q.; Geng, Z.; Duan, L.; Yang, M.; Guan, B. Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 2015, 186, 67–73. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Lao, Y.; Li, X.; Chen, F. A novel fed-batch strategy enhances lipid and astaxanthin productivity without compromising biomass of Chromochloris zofingiensis. Bioresour. Technol. 2020, 308, 123306. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Silveira, S.T.; Burkert, J.d.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K. Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Spiden, E.M.; Yap, B.H.; Hill, D.R.; Kentish, S.E.; Scales, P.J.; Martin, G.J. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef]
- Herrero, M.; Jaime, L.; Martín-Álvarez, P.J.; Cifuentes, A.; Ibáñez, E. Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J. Agric. Food Chem. 2006, 54, 5597–5603. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Jaime, L.; Santoyo, S.; Cifuentes, A.; Garcia-Blairsy Reina, G.; Senorans, F.; Ibáñez, E. Pressurized fluid extraction of bioactive compounds from Phormidium species. J. Agric. Food Chem. 2008, 56, 3517–3523. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Preto, M.A.; Sousa-Pinto, I.; Malcata, F.X. Gloeothece sp. as a Nutraceutical Source—An improved method of extraction of carotenoids and fatty acids. Mar. Drugs 2018, 16, 327. [Google Scholar] [CrossRef] [Green Version]
- Juin, C.; Chérouvrier, J.-R.; Thiéry, V.; Gagez, A.-L.; Bérard, J.-B.; Joguet, N.; Kaas, R.; Cadoret, J.-P.; Picot, L. Microwave-assisted extraction of phycobiliproteins from Porphyridium purpureum. Appl. Biochem. Biotechnol. 2015, 175, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.-M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Nogara, G.P.; Menezes, C.R.; Cichoski, A.J.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Identification of chlorophyll molecules with peroxyl radical scavenger capacity in microalgae Phormidium autumnale using ultrasound-assisted extraction. Food Res. Int. 2017, 99, 1036–1041. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Álvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. β-Carotene isomer composition of sub-and supercritical carbon dioxide extracts. Antioxidant activity measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef]
- Hosseini, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Liau, B.-C.; Shen, C.-T.; Liang, F.-P.; Hong, S.-E.; Hsu, S.-L.; Jong, T.-T.; Chang, C.-M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit. Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Macıas-Sánchez, M.; Mantell, C.; Rodrıguez, M.; de La Ossa, E.M.; Lubián, L.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Nannochloropsis gaditana. J. Food Eng. 2005, 66, 245–251. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Matias, A.A.; Nunes, A.V.; Pintado, M.E.; Duarte, C.M.; Malcata, F.X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [Green Version]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Montero, O.; Macías-Sánchez, M.D.; Lama, C.M.; Lubián, L.M.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M. Supercritical CO2 extraction of β-carotene from a marine strain of the cyanobacterium Synechococcus species. J. Agric. Food Chem. 2005, 53, 9701–9707. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M.; Lubián, L.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Zhang, R.; Marchal, L.; Lebovka, N.; Vorobiev, E.; Grimi, N. Two-step procedure for selective recovery of bio-molecules from microalga Nannochloropsis oculata assisted by high voltage electrical discharges. Bioresour. Technol. 2020, 302, 122893. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Saul Garcia-Perez, J.; Chandra, R.; Castillo-Zacarias, C.; Iqbal, H.; Parra-Saldívar, R. Methods for extraction of valuable products from microalgae biomass. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 245–263. [Google Scholar]
- Gong, M.; Li, X.; Bassi, A. Investigation of simultaneous lutein and lipid extraction from wet microalgae using Nile Red as solvatochromic shift probe. J. Appl. Phycol. 2018, 30, 1617–1627. [Google Scholar] [CrossRef]
- Aguilar-Machado, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Aguilar, C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ohmic heating processing conditions on color stability of fungal pigments. Food Sci. Technol. Int. 2017, 23, 338–348. [Google Scholar] [CrossRef]
- Araya, B.; Gouveia, L.; Nobre, B.; Reis, A.; Chamy, R.; Poirrier, P. Evaluation of the simultaneous production of lutein and lipids using a vertical alveolar panel bioreactor for three Chlorella species. Algal Res. 2014, 6, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Low, K.L.; Idris, A.; Yusof, N.M. Novel protocol optimized for microalgae lutein used as food additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.J.; Kumar, G.V.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Ahirwar, A.; Khan, M.J.; Sirotiya, V.; Mourya, M.; Rai, A.; Schoefs, B.; Marchand, J.; Varjani, S.; Vinayak, V. Pulsed Electric Field-Assisted Cell Permeabilization of Microalgae (Haematococcus pluvialis) for Milking of Value-Added Compounds. Bioenergy Res. 2022, 1–14. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Ooi, C.W.; Ong, H.C.; Ling, T.C.; Show, P.L. Extraction of natural astaxanthin from Haematococcus pluvialis using liquid biphasic flotation system. Bioresour. Technol. 2019, 290, 121794. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, D.; Ponrajan, A.; Bonnet, J.P.; Rocheford, T.; Ferruzzi, M.G. Carotenoid stability during dry milling, storage, and extrusion processing of biofortified maize genotypes. J. Agric. Food Chem. 2018, 66, 4683–4691. [Google Scholar] [CrossRef]
- Bell, T.; Alamzad, R.; Graf, B. Effect of pH on the chemical stability of carotenoids in juice. Proc. Nutr. Soc. 2016, 75, E94. [Google Scholar] [CrossRef] [Green Version]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, E.; Andujar-Sanchez, M.; Ortiz-Salmeron, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M.J.; Camara-Artigas, A.; Martinez-Rodriguez, S. Thermal and pH stability of the B-phycoerythrin from the red algae Porphyridium cruentum. Food Biophys. 2014, 9, 184–192. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef] [Green Version]
- Braga, A.R.C.; Figueira, F.d.S.; Silveira, J.T.d.; Morais, M.G.d.; Costa, J.A.V.; Kalil, S.J. Improvement of thermal stability of c-phycocyanin by nanofiber and preservative agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Selig, M.J.; Malchione, N.M.; Gamaleldin, S.; Padilla-Zakour, O.I.; Abbaspourrad, A. Protection of blue color in a spirulina derived phycocyanin extract from proteolytic and thermal degradation via complexation with beet-pectin. Food Hydrocoll. 2018, 74, 46–52. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Dunaliella: Biology, production, and markets. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 359–368. [Google Scholar]
- Paniagua-Michel, J. Microalgal nutraceuticals. In Handbook of Marine Microalgae; Elsevier: Amsterdam, The Netherlands, 2015; pp. 255–267. [Google Scholar]
- Grama, B.S.; Chader, S.; Khelifi, D.; Agathos, S.N.; Jeffryes, C. Induction of canthaxanthin production in a Dactylococcus microalga isolated from the Algerian Sahara. Bioresour. Technol. 2014, 151, 297–305. [Google Scholar] [CrossRef]
- Mulders, K.J.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]


| Pigment | Common Microalgal Source | Bioactivities Known | References |
|---|---|---|---|
| Chlorophylls | Chlorella sp. Monoraphidium dybowskii Scenedesmus dimorphus Chlamydomonas reinhardtii Pavlova lutheri Chlorella vulgaris | • Improving immune system • High antioxidant • Anti-carcinogen | [9,10] |
| β-Carotene | Dunaliella salina Dunaliella bardawil | • Anticancer • Antioxidant • Suppression of cholesterol synthesis • Vitamin A activity • Protect skin against sunburn • Prevent coronary artery diseases, fatty liver disease, type-2 diabetes, insulin resistance, age-related macular degeneration, and ultraviolet-induced skin cancer and oral carcinomas | [10,11,12,13,14] |
| Astaxanthin | Haematococcus pluvialis Chlorella zofingiensis Chlorella sp. | • Antioxidant and photoprotector • Anti-inflammatory, antineoplastic and anticancer • Antimicrobial • Anti-hyperlipidemia, increase serum adiponectin • Beneficial effects on blood rheology and metabolic syndrome | [11,15,16,17,18,19] |
| Lutein | Chlorella protothecoides Muriellopsis sp. Scenedesmus almerienses Dunaliella salina Dunaliella tertiolecta Brassica oleracea Spinacia oleracea Actinidia deliciosa | • Antioxidant, prevent the production of free radicals • Immunity strengthens • Anti-inflammatory • Anti-atherogenic and antihypertensive • Cytoprotection against alcohol-induced liver injury • Increase visual sensitivity and filter the harmful blue light • Potent neuroprotection • Prevention of cataracts, age-related macular degeneration, skeletal ischemia, hepatotoxicity and cardiovascular diseases | [10,20,21,22,23] |
| Fucoxanthin | Cylindrotheca closterium Phaeodactylum tricornutum Isochrysis galbana Mallomonas sp. Nitzschia laevis Odontella aurita Chaetoceros sp. | • Stimulate levels of cytokines • Reduce blood triglyceride concentration • Anti-inflammatory, inhibit pro-inflammatory factors, improve phagocytic and microbicidal capacity • Antioxidant, decrease oxidative damage to lipids/proteins • Antineoplastic, inhibit the growth of human leukemia cells and neuroblastoma cells • Anti-obesity | [10,17,24] |
| Phycobiliproteins | Spirulina platensis Geitlerinema Porphyridium sp. | • Cytotoxicity • Apoptosis • Anti-alzhelmeric activity • Antioxidant | [10,11,12,16] |
| Pigment | Color | Applications | Target Foods |
|---|---|---|---|
| Chlorophylls | Green | • Food ingredients • Food colorant | Beverages, fruit juices, pasta, dairy products, sweetener, preparations, soups |
| β-Carotene | Red orange | • Food colorant • Feed additive • Vitamin A source | Butter, margarine, cakes, cheese, dairy products, soft drinks, baked products, canned foods, confectioneries, health condiments, dairy products |
| Astaxanthin | Red orange | • Food colorant • Feed additive • Dietary supplements • Functional foods • Cosmetics | Foods, nutraceuticals, pet foods |
| Lutein | Yellow orange | • Food colorant • Functional foods | Dairy products, soft drinks, confections, flavorings, salads, pastries, confectionery, infant formula |
| Fucoxanthin | Yellow brown | • Feed additive • Dietary supplements • Cosmetics | Egg yolk, butter, pie, cakes and other baked foods |
| Phycocyanin, Allophycocyanin | Blue | • Food colorant • Fluorescent markers | Sweets, ice cream, food, nutraceuticals, chewing gum, jellies |
| Phycoerythrin | Red | • Food colorant | Sweets, ice cream, food, nutraceuticals, chewing gum, jellies |
| Extraction Method | Microalgae | Target Pigments | Solvent | Temperature (℃) | Pressure (Bar) | References |
|---|---|---|---|---|---|---|
| Solvent extraction | Isochrysis galbana | Fucoxanthin | ethanol | RT | [35] | |
| H. pluvialis | Astaxanthin | ethanol:ethyl acetate (1:1, v:v) | RT | [115] | ||
| S. platensis | PBP | sodium phosphate | 25 | [116] | ||
| Enzymatic HPH | A. platensis | APB | lysozyme + surfactants | 37 | [117] | |
| Nannochloropsis sp. | Carotenoids | 3% w/w NaCl | n.s. | 1380 | [118] | |
| Chlorella sp. | Carotenoids | 3% w/w NaCl | n.s. | 1070 | [118] | |
| Tetraselmis sp. | Carotenoids | 3% w/w NaCl | n.s. | 170 | [118] | |
| Nannochloropsis sp. | Violoxanthin, antheraxanthin, zeaxanthin, β-carotene | water/recovered with hexane:isopropanol (3:2, v:v) | n.s. | 1000 | [119] | |
| PLE | C. vulgaris | Lutein | ethanol:water (9:1, v:v) | 148 | 100 | [120] |
| β-carotene | ethanol:water (9:1, v:v) | 117 | 100 | [120] | ||
| Chlorophyll a | ethanol:water (9:1, v:v) | 173 | 100 | [120] | ||
| Chlorophyll b | ethanol:water (9:1, v:v) | 170 | 100 | [120] | ||
| D. salina | β-carotene | ethanol | 160 | 100 | [121] | |
| Phormidium spp. | Carotenoids | ethanol | 150 | 100 | [122] | |
| CPSE | Gloeothece sp. | Carotenoids | ethanol | 60 | 180 | [123] |
| MAE | Porphyridium purpureum | PE | water | 40 | [124] | |
| PC/APC | water | 100 | [124] | |||
| Cylindrotheca closterium | Fucoxanthin | acetone | 56 | [125] | ||
| UAE | A. platensis | β-carotene | ethanol | 30 | [115] | |
| H. pluvialis | Carotenoids | heptane | 41.1 | [126] | ||
| PEF | C. vulgaris | Carotenoids | citrate- phosphate McIlvaine | n.s. | [127] | |
| A. platensis | C-PC | water | 40 | [128] | ||
| Nostoc commune | C-PC | water | 40 | [129] | ||
| Porphyridium cruentum | PC | citrate-phosphate McIlvaine | 20–30 | [130] | ||
| PEF pre-treatment | Nannochloropsis spp. | Carotenoids | recovered in DMSO | 20 | [131] | |
| H. pluvialis | Astaxanthin | recovered with ethanol | 20 | [132] | ||
| SC-CO2 | D. salina | β-carotene | SC-CO2 | 27.5 | 443 | [133] |
| D. salina | Carotenoids | SC-CO2 | 30 | 400 | [134] | |
| Nannochloropsis oculata | Carotenoids | SC-CO2 + Ethanol | 50 | 350 | [135] | |
| Nannochloropsis gaditana | Carotenoids, chlorophylls | SC-CO2 | 60 | 400 | [136] | |
| Scenedesmus obliquus | Carotenoids | SC-CO2 | 60 | 250 | [137] | |
| H. pluvialis | Astaxanthin, lutein | SC-CO2 | 50 | 550 | [138] | |
| H. pluvialis | Astaxanthin | SC-CO2 + Ethanol | 60 | 300 | [139] | |
| Synechococcus sp. | β-carotene | SC-CO2 | 50 | 358 | [140] | |
| Synechococcus sp. | Carotenoids | SC-CO2 | 50 | 300 | [141] | |
| Laser | N. oculata | Carotenoids, chlorophyll a | water | n.s | [142] | |
| HVED | N. oculata | Chlorophylls, carotenoids | water | 20–30 | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. https://doi.org/10.3390/md21020082
Sun H, Wang Y, He Y, Liu B, Mou H, Chen F, Yang S. Microalgae-Derived Pigments for the Food Industry. Marine Drugs. 2023; 21(2):82. https://doi.org/10.3390/md21020082
Chicago/Turabian StyleSun, Han, Yuxin Wang, Yongjin He, Bin Liu, Haijin Mou, Feng Chen, and Shufang Yang. 2023. "Microalgae-Derived Pigments for the Food Industry" Marine Drugs 21, no. 2: 82. https://doi.org/10.3390/md21020082