Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy
Abstract
:1. Introduction
2. Overview of Fucoidans
2.1. Structural Characteristics of Fucoidans
| Name of Algae | Place of Collection | Extraction Method | Yield (%) | Monosaccharide Composition (%) | Molecular Weight (kDa) | Ref. |
|---|---|---|---|---|---|---|
| Ascophyllum nodosum | ND | Acid hydrolysis, centrifugal partition, chromatography. | ND | Fuc: ND. | ND. | [50] |
| Cladosiphon navae-caledoniae | Daiichi Sangyo Corporation (Osaka, Japan) | ND | HMWF: 85, LMWF: 72 | ND LMWF: Fuc: 73, xyl: 12, man: 7 | LMWF: <500. | [33] |
| Fucus serratus, Fucus vesiculosus, Ascophyllum nodosum | Coast of Aberystwyth at low tide, UK | CaCl2 extraction | 6.0 wt, 9.8 wt, 8.0 wt | fuc:18–28 wt, sulphate: 30–40 wt; fuc: 26–39 wt, sulphate: 9–35 wt; fuc: 35–46 wt, sulphate: 6–22 wt. | 1608; 1364 1374 | [31] |
| Fucus vesiculosus | Purchased from Sigma | ND | ND | Fuc: 97, gal: ND, xyl: ND. | 100 | [37] |
| Fucus vesiculosus | Fucoidan extract 1 (FE1): from Marinova; FE2 and FE3 from Sigma-Aldrich | ND | FE1: 52.5 FE2: 52.2 FE3: 50.5 | FE1: Fuc: 73.1, xyl: 8.0, man: 1.3, gal: 3.5, glu: 0.7. FE2: Fuc: 79.1 xyl: 3.9, man: 0.8, gal: 5.5, glu: 0.8. FE3: Fuc: 71.2, xyl: 5.3, man: 1.5, gal: 5.4, glu: 1.3. | FE1: 91 FE2: 60 FE3: 42 | [32] |
| Fucus evanescens | Littoral of Iturup island (Kuril Islands) | CaCl2 extraction | Fraction F1: 3.9, Fraction F2: 2.6, Fraction F3: 21.4, Fraction F4: 47.4 Fraction F5: 4.5 | F1: fuc: 35.4, xyl: 6.1, man: 0.8, glu: 4.0. F2: fuc: 10.7, xyl: 17.4, gal: 3.0, man: 3.7, glu: 1.1. F3: fuc: 33.2, xyl:8.1, gal:4.5, man: 3.5 F4: fuc: 58.7, xyl: 1.6, gal: 1.6. F5: fuc: 34.0, xyl: 3.8, gal: 5.4. | ND. | [51] |
| Laminaria Saccharina, Laminaria digitata, Cladosiphon okamuranus, Fucus evanescens, Fucus vesiculosus, Fucus serratus, Fucus distichus, Fucus spiralis, Ascophyllum nodosum | ND | CaCl2 extraction | ND | Fuc: 36.7 (w/w), xyl: 1.2 (w/w), man: 1.0 (w/w), glu: 2.2 (w/w), gal: 4.6 (w/w). Fucose: 30.1 (w/w), xyl: 1.9 (w/w), man: 1.7 (w/w), glu: 1.4 (w/w), gal: 6.3 (w/w). Fuc: 30.9 (w/w), xyl: 0.7 (w/w), glu: 2.2 (w/w). Fuc: 58.7 (w/w), xyl: 1.6 (w/w), gal: 1.6 (w/w). Fuc: 26.1 (w/w), xyl: 2.4 (w/w), man: 3.1 (w/w), glu: 2.2 (w/w), gal: 5.0 (w/w). Fuc: 24.8 (w/w), xyl: 2.4 (w/w), man: 2.1 (w/w), glu: 2.0 (w/w), gal: 4.8 (w/w). Fuc: 40.8 (w/w), xyl: 0.8 (w/w), gal: 0.8 (w/w). Fuc: 33.0 (w/w), xyl: 2.8 (w/w), man: 1.4 (w/w), glu: 1.2 (w/w), gal: 3.0 (w/w). Fuc: 26.6 (w/w), xyl: 4.4 (w/w), man: 2.6 (w/w), glu: 1.1 (w/w), gal: 4.7 (w/w). | 200–500 | [34] |
| Laminaria hyperborea | ND | ND | ND | Fuc: 97.8, gal: 2.2, glu: ND. | 469.2 | [45] |
| Undaria pinnatifida | Great Barrier Island, Port Underwood New Zealand | CaCl2 extraction | Increased from July (25.4–26.3) to September (57.3–69.9). | Crude fucoidan (F0): L-fuc: 39.24, D-gal: 26.48, D-xyl: 28.85, D-man: 5.04, -D-glu: 0.95. Commercial fucoidan: L-fuc: 87.12, D-gal: 5.69, D-xyl: 4.85, D-man: 1.39, -D-glu: 0.94. Fucoidan fraction F1: L-fuc: 48.51, D-gal: 37.86, D-xyl: 3.74, D-man: 6.97, -D-glu: 2.91. F2: L-fuc: 53.21, D-gal: 42.12, D-xyl: 1.15, D-man: 2.24, -D-glu: 1.28. F3: L-fuc: 59.71, D-gal: 28.74, D-xyl: 1.58, D-man: 7.19 -D-glu: 2.77. | F0: 171 F1: 81 F2: 22 F3: 27 | [6] |
| Undaria pinnatifida | Five fucoidans purchased from Sigma Aldrich, Anhui Minmetals Development I/E Co. Ltd., Matakana SuperFoods, Glycomix UK, and Leili Ltd | CaCl2 extraction | Undaria pinnatifida fucoidan (S): ≥95, Crude fucoidan (S1): ND, S2: 75.5 S3: ND, S4: 75 S5: ND | Fucoidan(S): fuc: 27.44, gal: 25.34, Fraction S1: fuc: 19.50, gal: 21.20. Fraction S2: glu: 96.71, Fraction S3: fuc: 13.83, gal: 13.24. Fraction S4: fuc: 20.35, gal: 19.26. Fraction S5: fuc: 19.23, gal: 21.00, glu: 6.38. | S: 440 S1: 440–2000 S2: <2000 S3: 38.9–440 S4: 35.2–440 S5: 2 | [52] |
| Undaria Pinnatifida | From Sigma Aldrich | CaCl2 extraction | Undaria pinnatifida fucoidan: ≥95 LMWF: ND | ND. | ND LMWF: <10 | [48] |
| Undaria Pinnatifida Fucus vesiculosus | Port Underwood, New Zealand | CaCl2 extraction | Sporophyll derived from farm 327: 69, Sporophyll derived from farm 106: 57.28 | Crude fucoidan (F0): fuc: 39.24, xyl: 28.85, gal: 26.48, man: 5.04, glu: 0.95, F1: fuc: 48.51, xyl: 3.74, gal: 37.86, man: 6.97, glu: 2.91. F2: fuc: 53.21, xyl: 1.15, gal: 42.12, man: 2.24, glu: 1.28. Fraction F: fuc: 59.71, xyl: 1.58, gal: 28.74, man: 7.19, glu: 2.77, Fucoidan (Sigma): fuc: 87.12, xyl: 4.85, gal: 5.69, man: 1.39, glu: 0.94. | F0: >150 F1: 81, F2: 22 F: 27 54 | [53] |
2.2. Pharmacological Actions of Fucoidans
2.3. Pharmacokinetics of Fucoidans
2.4. Biomedical Usages of Fucoidans
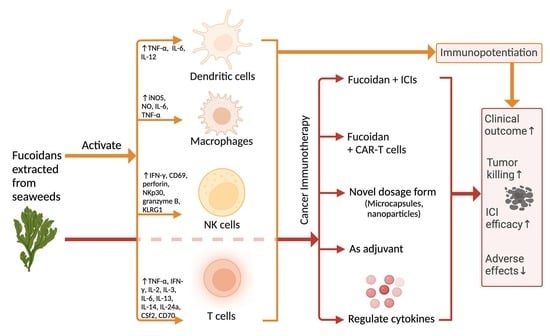
3. Immunopotentiating Effects of Fucoidans
3.1. Overall Effects of Fucoidans on the Immune System
3.2. Immunological Effect of Fucoidans on T Cells
3.3. Immunological Effect of Fucoidan on Dendritic Cells
3.3.1. Fucoidan Activates the Maturation of DCs via Toll-like Receptors
3.3.2. Fucoidans Activate the Maturation of DCs via Scavenger Receptor Type A (SR-A)
3.4. Immunological Effect of Fucoidan on Macrophages
3.5. Immunological Effect of Fucoidan on NK Cells
3.6. Factors Influencing Fucoidan-Activated Immune Cells
4. Relevance to Cancer Immunotherapy
4.1. Fucoidan Potentiates Cancer Immunotherapy
4.2. Fucoidan Enhances the Efficacy of Immunotherapy via Novel Dosage Forms
4.3. Fucoidan Enhances T Cell Activation via Increasing IL-2, IFN-, TNF-
4.4. Fucoidan Enhance Immunotherapy through Regulating Cytokines Released by Macrophages and NK Cells
4.5. Possible Combined Use of Fucoidan with Immunotherapeutic Products
5. Relevant Clinical Trials-Anticancer and Immunomodulation Related Trials
| Clinical Trial Number | Starting Year (Status) | Description | Results |
|---|---|---|---|
| NCT04342949 | 2018 (unknown) | A double-blind, randomized, placebo-controlled, parallel study investigated fucoidan’s auxiliary effects in patients with locally advanced rectal cancer who received a combined radio/chemotherapy before surgery. They aimed to observe whether fucoidan can improve the quality of life of these patients receiving the neoadjuvant CCRT. | No |
| NCT04066660 | 2019 (Recruiting) | A randomized, double-blind, controlled trial evaluated oligo-fucoidan’s efficacy (500–800 Da) in patients with metastatic colorectal cancer. They have also observed whether fucoidan can improve the quality of life and prolong the overall survival rate of these patients. | Yes [164] |
| NCT04597476 | 2020 (Recruiting) | A randomized, double-blind phase II trial that evaluated fucoidan’s clinical effect and safety in patients with stage III/IV head and neck squamous cell carcinoma. | No |
| NCT03130829 | 2019 (Withdrawn) | A pilot, randomized, double-blind, multicenter study evaluated whether orally administered oligo-fucoidan can improve the quality of life in patients receiving platinum-based chemotherapy with NSCLC. | No |
| NCT02875392 | 2016 (Completed) | A randomized, double-blind, parallel study demonstrated that fucoidan improves the metabolic profiles of patients with non-alcoholic fatty liver disease (NAFLD). | Yes [166] |
| NCT05437887 | 2022 (Not yet recruiting) | An open-label, prospective, single group study evaluated the effects of fucoidan on the gut microbiota in the patients of atopic dermatitis before and after fucoidan treatment. | No |
| NCT05461508 | 2023 (Recruiting) | An open-label, randomized, parallel study investigates the effects of the combination treatment (fucoidan and Vonoprazan) on Helicobacter Pylori eradication rate and gastrointestinal flora. | No |
| NCT03422055 | 2018 (Unknown) | An open-label, single-group phase I study that evaluated the tolerance, biodistribution, and dosimetry of fucoidan radiolabeled by Technetium-99 m in patients. | No |
| ACTRN126 16000417482 | 2016 (Completed recruitment) | A randomized, double-blind, placebo-controlled, cross-over phase I/II trial that investigated the measurement, modulation, and estimation of net endogenous non-carbonic acid production using the Australian food database following the administration of alkaline supplements in healthy adults. | N/A |
| ACTRN126 15000673549 | 2015 (Completed recruitment) | An open-label, non-randomized, single-group phase IV trial investigating the interaction between two systemic complementary and alternative medicines and standard therapy in patients with active breast cancer malignancy. | Yes [24] |
| ACTRN126 05000021673 | 2005 (Recruiting) | A non-randomized, double-blind, parallel phase I/II study that evaluated the effects of natural seaweed fucoidan (GFS) on the modulation of the immune system and the mobilization/release of hematopoietic progenitor stem cells from bone marrow to the peripheral blood. | No |
| ACTRN1262 1000872831 | 2021 (Not yet recruiting) | A randomized, double-blind, crossover trial determines whether daily fucoidan supplementation can upregulate immune biomarkers during three weeks of intensified exercise training in healthy, recreationally active adults. | No |
| ACTRN1260 5000021673 | 2005 (Recruiting) | A non-randomized, double-blind, parallel phase I/II study that evaluated the effects of natural seaweed fucoidan (GFS) on the modulation of the immune system and the mobilization/release of hematopoietic progenitor stem cells from bone marrow to the peripheral blood. | No |
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Tomori, M.; Nagamine, T.; Miyamoto, T.; Iha, M. Evaluation of the Immunomodulatory Effects of Fucoidan Derived from Cladosiphon Okamuranus Tokida in Mice. Mar. Drugs 2019, 17, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Yang, H.-W.; Choi, C.S.; Jeon, Y.-J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Kiselevskiy, M.V.; Anisimova, N.Y.; Ustyuzhanina, N.E.; Vinnitskiy, D.Z.; Tokatly, A.I.; Reshetnikova, V.V.; Chikileva, I.O.; Shubina, I.Z.; Kirgizov, K.I.; Nifantiev, N.E. Perspectives for the Use of Fucoidans in Clinical Oncology. Int. J. Mol. Sci. 2022, 23, 11821. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, K.R.; Rajaram, R.; Sivakumar, S.R. A critical review on pharmacological properties of marine macroalgae. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Agency, E.M. Assessment Report on Fucus vesiculosus L., thallus; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Kylin, H. Zur Biochemie der Meeresalgen. 1913. Available online: https://www.degruyter.com/document/doi/10.1515/bchm2.1913.83.3.171/html (accessed on 15 December 2022).
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [Green Version]
- Deniaud-Bouet, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Herve, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Pan, W.; Wang, M.; Lu, Y.; Zhang, J.; Fang, Z.; Zhang, X.; Ji, Y.; Bei, J.-X.; et al. Fucoidan-Supplemented Diet Potentiates Immune Checkpoint Blockage by Enhancing Antitumor Immunity. Front. Cell Dev. Biol. 2021, 9, 733246. [Google Scholar] [CrossRef]
- Fucoidan, N.R.I.O. High-Molecular Weight Fucoidan & Low-Molecular Weight Fucoidan. Available online: https://www.fucoidan-life.com/en/info_fucoidan/fucoidan/ (accessed on 3 February 2023).
- McGowan, E.; Lin, Q.; Ma, G.; Yin, H.; Chen, S.; Lin, Y. PD-1 disrupted CAR-T cells in the treatment of solid tumors: Promises and challenges. Biomed. Pharmacother. 2020, 121, 109625. [Google Scholar] [CrossRef]
- Park, H.B.; Lim, S.M.; Hwang, J.; Zhang, W.; You, S.; Jin, J.O. Cancer immunotherapy using a polysaccharide from Codium fragile in a murine model. Oncoimmunology 2020, 9, 1772663. [Google Scholar] [CrossRef]
- Park, H.B.; Hwang, J.; Lim, S.M.; Zhang, W.; Jin, J.O. Dendritic cell-mediated cancer immunotherapy with Ecklonia cava fucoidan. Int. J. Biol. Macromol. 2020, 159, 941–947. [Google Scholar] [CrossRef]
- Zhang, W.; Hwang, J.; Yadav, D.; An, E.-K.; Kwak, M.; Lee, P.C.-W.; Jin, J.-O. Enhancement of Immune Checkpoint Inhibitor-Mediated Anti-Cancer Immunity by Intranasal Treatment of Ecklonia cava Fucoidan against Metastatic Lung Cancer. Int. J. Mol. Sci. 2021, 22, 9125. [Google Scholar] [CrossRef]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients With Breast Cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phull, A.R.; Kim, S.J. Fucoidan from Undaria pinnatifida regulates type II collagen and COX-2 expression via MAPK and PI3K pathways in rabbit articular chondrocytes. Biologia 2017, 72, 1362–1369. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Lan, Y.; Liu, J.; Zhang, F.; Zhang, L.; Li, B.; Zhao, X. The structure property and endothelial protective activity of fucoidan from Laminaria japonica. Int. J. Biol. Macromol. 2017, 105, 1421–1429. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanases—Focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Ferreira, A.S.; Novoa-Carballal, R.; Nunes, C.; Pashkuleva, I.; Neves, N.M.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Silva, T.H. The Key Role of Sulfation and Branching on Fucoidan Antitumor Activity. Macromol. Biosci. 2017, 17, 1600340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013, 77, 235–242. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, X.; Yuan, P.; Cai, S.; Bao, J.; Zhao, Y.; Aimaier, A.; Aipire, A.; Lu, J.; Li, J. In Vitro and In Vivo Dendritic Cell Immune Stimulation Effect of Low Molecular Weight Fucoidan from New Zealand Undaria pinnatifida. Mar. Drugs 2022, 20, 197. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, N.; Suo, Q.; Wang, J.; Yue, Y.; Geng, L.; Zhang, Q. Fucoidan, as an immunostimulator promotes M1 macrophage differentiation and enhances the chemotherapeutic sensitivity of capecitabine in colon cancer. Int. J. Biol. Macromol. 2022, 222, 562–572. [Google Scholar] [CrossRef]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.-M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [Green Version]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. 11–Fucoidan and Its Health Benefits. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 223–238. [Google Scholar] [CrossRef]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a noval amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterization of Fucoidan from Laminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure–Function Relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Ushakova, N.A.; Usov, A.I.; Kiselevskiy, M.V.; Nifantiev, N.E. Fucoidans: Pro- or antiangiogenic agents? Glycobiology 2014, 24, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.; Trangle, S.S.; Li, Y.; White, W.L.; Li, J.; Ying, T.; Kong, Q.; Zhao, Y.; Lu, J. Investigation of Different Molecular Weight Fucoidan Fractions Derived from New Zealand Undaria pinnatifida in Combination with GroA Therapy in Prostate Cancer Cell Lines. Mar. Drugs 2018, 16, 454. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.J.; You, D.J.; Lee, K.W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef] [Green Version]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, K.K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.N.; Merien, F.; Xie, M.; Kong, Q.; Li, J.; et al. Fucoidan Extracted from the New Zealand Undaria pinnatifida-Physicochemical Comparison against Five Other Fucoidans: Unique Low Molecular Weight Fraction Bioactivity in Breast Cancer Cell Lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, W.; Wang, S.K.; Liu, T.; Hamid, N.; Li, Y.; Lu, J.; White, W.L. Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Front. Nutr. 2014, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- van Weelden, G.; Bobinski, M.; Okla, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Jin, J.O.; Yadav, D.; Madhwani, K.; Puranik, N.; Chavda, V.; Song, M. Seaweeds in the Oncology Arena: Anti-Cancer Potential of Fucoidan as a Drug-A Review. Molecules 2022, 27, 6032. [Google Scholar] [CrossRef]
- Guo, R.; Deng, M.; He, X.; Li, M.; Li, J.; He, P.; Liu, H.; Li, M.; Zhang, Z.; He, Q. Fucoidan-functionalized activated platelet-hitchhiking micelles simultaneously track tumor cells and remodel the immunosuppressive microenvironment for efficient metastatic cancer treatment. Acta Pharm. Sin. B 2022, 12, 467–482. [Google Scholar] [CrossRef]
- do-Amaral, C.C.F.; Pacheco, B.S.; Seixas, F.K.; Pereira, C.M.P.; Collares, T. Antitumoral effects of fucoidan on bladder cancer. Algal Res. 2020, 47, 101884. [Google Scholar] [CrossRef]
- Zhang, Z.; Till, S.; Jiang, C.; Knappe, S.; Reutterer, S.; Scheiflinger, F.; Szabo, C.M.; Dockal, M. Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thromb. Haemost. 2014, 111, 429–437. [Google Scholar] [CrossRef]
- Mansour, M.B.; Balti, R.; Yacoubi, L.; Ollivier, V.; Chaubet, F.; Maaroufi, R.M. Primary structure and anticoagulant activity of fucoidan from the sea cucumber Holothuria polii. Int. J. Biol. Macromol. 2019, 121, 1145–1153. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Church, F.C.; Meade, J.B.; Treanor, R.E.; Whinna, H.C. Antithrombin Activity of Fucoidan: The interaction of fucoidan with heparin cofactor II, antithrombin III, and thrombin. J. Biol. Chem. 1989, 264, 3618–3623. [Google Scholar] [CrossRef]
- Mauray, S.; de Raucourt, E.; Talbot, J.C.; Dachary-Prigent, J.; Jozefowicz, M.; Fischer, A.M. Mechanism of factor IXa inhibition by antithrombin in the presence of unfractionated and low molecular weight heparins and fucoidan. Biochim. Biophys. Acta 1998, 1387, 184–194. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.Y.; Kang, H.J.; Kim, H.J.; Lee, J. Fucoidan/FGF-2 induces angiogenesis through JNK- and p38-mediated activation of AKT/MMP-2 signalling. Biochem. Biophys. Res. Commun. 2014, 450, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.-M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chabut, D.; Fischer, A.M.; Helley, D.; Colliec, S. Low molecular weight fucoidan promotes FGF-2-induced vascular tube formation by human endothelial cells, with decreased PAI-1 release and ICAM-1 downregulation. Thromb. Res. 2004, 113, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourão, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on Antiviral and Immuno-Regulation Activity of Low Molecular Weight Fucoidan from Laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Lin, H.T.; Chen, C.C.; Chiao, D.J.; Chang, T.Y.; Chen, X.A.; Young, J.J.; Kuo, S.C. Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice. Int. J. Biol. Macromol. 2021, 193, 1885–1897. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.-Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef]
- Yamamoto, I.; Takahashi, M.; Suzuki, T.; Seino, H.; Mori, H. Antitumor effect of seaweeds. IV. Enhancement of antitumor activity by sulfation of a crude fucoidan fraction from Sargassum kjellmanianum. Jpn. J. Exp. Med. 1984, 54, 143–151. [Google Scholar]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan Extract Induces Apoptosis in MCF-7 Cells via a Mechanism Involving the ROS-Dependent JNK Activation and Mitochondria-Mediated Pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Abudabbus, A.; Badmus, A.J.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of Fucoidan and Chemotherapeutic Agent Combinations on Malignant and Non-malignant Breast Cell Lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, Q.; Shi, X.; Zheng, Q.; Chen, L.; Sun, Y. Advances in plant-derived natural products for antitumor immunotherapy. Arch. Pharm. Res. 2021, 44, 987–1011. [Google Scholar] [CrossRef]
- Banafa, A.M.; Roshan, S.; Liu, Y.Y.; Chen, H.J.; Chen, M.J.; Yang, G.X.; He, G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 717–724. [Google Scholar] [CrossRef]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hu, C.-H.; Shu, D.T.F.; Lu, M.-K. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018, 432, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Lu, M.K.; Leng, P.J.; Tsao, S.M.; Wu, Y.C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Sun, L.; Wei, X.; Lu, H.; Tan, Y.; Sun, Z.; Jiang, J. Antitumor effect and molecular mechanism of fucoidan in NSCLC. BMC Complement Med. 2021, 21, 25. [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cong, Q.; Du, Z.; Liao, W.; Zhang, L.; Yao, Y.; Ding, K. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016, 382, 44–52. [Google Scholar] [CrossRef]
- Danforth, D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res./Rev. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, S.C.; Chan, K.T.; Ke, Y.; Xue, B.; Sin, F.W.; Zeng, C.; Xie, Y. Fucoidin enhances dendritic cell-mediated T-cell cytotoxicity against NY-ESO-1 expressing human cancer cells. Biochem. Biophys. Res. Commun. 2010, 392, 329–334. [Google Scholar] [CrossRef]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune Activation of RAW264.7 Macrophages by Low Molecular Weight Fucoidan Extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.; Fernando, I.P.; Kim, E.A.; Ahn, G.; Jee, Y.; Jeon, Y.J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ryu, J.M.; Han, Y.-S.; Zia, M.F.; Kwon, H.Y.; Noh, H.; Han, H.J.; Lee, S.H. Fucoidan improves bioactivity and vasculogenic potential of mesenchymal stem cells in murine hind limb ischemia associated with chronic kidney disease. J. Mol. Cell. Cardiol. 2016, 97, 169–179. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Ahmad, T.; Eapen, M.S.; Ishaq, M.; Park, A.Y.; Karpiniec, S.S.; Stringer, D.N.; Sohal, S.S.; Fitton, J.H.; Guven, N.; Caruso, V.; et al. Anti-Inflammatory Activity of Fucoidan Extracts In Vitro. Mar. Drugs 2021, 19, 702. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Ahn, C.; Kang, B.-T.; Kang, J.-H.; Jeung, E.-B.; Yang, M.-P. Fucoidan suppresses excessive phagocytic capacity of porcine peripheral blood polymorphonuclear cells by modulating production of tumor necrosis factor-alpha by lipopolysaccharide-stimulated peripheral blood mononuclear cells. Res. Vet. Sci. 2018, 118, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; Lee, J.S.; et al. Fucoidan Purified from Sargassum polycystum Induces Apoptosis through Mitochondria-Mediated Pathway in HL-60 and MCF-7 Cells. Mar Drugs 2020, 18, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, K.; Zhao, Y. Fucoidan inhibits amyloid-β-induced toxicity in transgenic Caenorhabditis elegans by reducing the accumulation of amyloid-β and decreasing the production of reactive oxygen species. Food Funct. 2018, 9, 552–560. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Zhan, E.; Chu, F.; Zhao, T.; Chai, Y.; Liang, H.; Song, S.; Ji, A. Determination of fucoidan in rat plasma by HPLC and its application in pharmacokinetics. Pak. J. Pharm. Sci. 2020, 33, 1–9. [Google Scholar]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sun, D.; Zhao, X.; Jin, W.; Wang, J.; Zhang, Q. Microanalysis and preliminary pharmacokinetic studies of a sulfated polysaccharide from Laminaria japonica. Chin. J. Oceanol. Limnol. 2016, 34, 177–185. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P. Is the transformation of fucoidans in human body possible? Int. J. Biol. Macromol. 2020, 142, 778–781. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Hu, B.; Liang, H.; Song, S.; Ji, A. Study on Absorption Mechanism and Tissue Distribution of Fucoidan. Molecules 2020, 25, 1087. [Google Scholar] [CrossRef] [Green Version]
- Marinova Pty. Ltd. Determination of the Generally Recognized as Safe (Gras) Status of Fucoidan from Fucus Vesiculosus as a Food Ingredient; Food and Drug Administration: College Park, MD, USA, 2016.
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, I.; Ishizaka, S. Polysaccharides with sulfate groups are human T-cell mitogens and murine polyclonal B-cell activators (PBAs). I. Fucoidan and heparin. Cell. Immunol. 1982, 74, 162–171. [Google Scholar] [CrossRef]
- Zapopozhets, T.S.; Besednova, N.N.; Loenko Iu, N. [Antibacterial and immunomodulating activity of fucoidan]. Antibiot. Khimioter. 1995, 40, 9–13. [Google Scholar]
- Liu, J.N.; Yoshida, Y.; Wang, M.Q.; Okai, Y.; Yamashita, U. B cell stimulating activity of seaweed extracts. Int. J. Immunopharmacol. 1997, 19, 135–142. [Google Scholar] [CrossRef]
- Chiang, C.-S.; Lin, Y.-J.; Lee, R.; Lai, Y.-H.; Cheng, H.-W.; Hsieh, C.-H.; Shyu, W.-C.; Chen, S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.-O. Fucoidan from Macrocystis pyrifera Has Powerful Immune-Modulatory Effects Compared to Three Other Fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabarsa, M.; Dabaghian, E.H.; You, S.; Yelithao, K.; Cao, R.; Rezaei, M.; Alboofetileh, M.; Bita, S. The activation of NF-κB and MAPKs signaling pathways of RAW264.7 murine macrophages and natural killer cells by fucoidan from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2020, 148, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Zhang, W.; An, E.K.; Park, H.B.; Hwang, J.; Dhananjay, Y.; Kim, S.J.; Eom, H.Y.; Oda, T.; Kwak, M.; Lee, P.C.; et al. Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo. Int. J. Biol. Macromol. 2021, 185, 111–121. [Google Scholar] [CrossRef]
- Yang, M.; Ma, C.; Sun, J.; Shao, Q.; Gao, W.; Zhang, Y.; Li, Z.; Xie, Q.; Dong, Z.; Qu, X. Fucoidan stimulation induces a functional maturation of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2008, 8, 1754–1760. [Google Scholar] [CrossRef]
- Jin, J.O.; Park, H.Y.; Xu, Q.; Park, J.I.; Zvyagintseva, T.; Stonik, V.A.; Kwak, J.Y. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 2009, 113, 5839–5847. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-κB pathway in M2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef]
- Makarenkova, I.D.; Logunov, D.Y.; Tukhvatulin, A.I.; Semenova, I.B.; Besednova, N.N.; Zvyagintseva, T.N. Interactions between sulfated polysaccharides from sea brown algae and Toll-like receptors on HEK293 eukaryotic cells in vitro. Bull. Exp. Biol. Med. 2012, 154, 241–244. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, Y.; Kim, G.H.; Cho, H. Undaria pinnatifida Fucoidan-Rich Extract Recovers Immunity of Immunosuppressed Mice. J. Microbiol. Biotechnol. 2020, 30, 439–447. [Google Scholar] [CrossRef]
- Park, A.Y.; Nafia, I.; Stringer, D.N.; Karpiniec, S.S.; Fitton, J.H. Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Mar. Drugs 2022, 20, 12. [Google Scholar] [CrossRef]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Moon, S.-Y.; Joo, H.-G. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem. Toxicol. 2014, 68, 234–238. [Google Scholar] [CrossRef]
- Reis e Sousa, C. Toll-like receptors and dendritic cells: For whom the bug tolls. Semin. Immunol. 2004, 16, 27–34. [Google Scholar] [CrossRef]
- Gordon, S. Pattern recognition receptors: Doubling up for the innate immune response. Cell 2002, 111, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Kaliński, P.; Hilkens, C.M.U.; Wierenga, E.A.; Kapsenberg, M.L. T-cell priming by type-1and type-2 polarized dendritic cells: The concept of a third signal. Immunol. Today 1999, 20, 561–567. [Google Scholar] [CrossRef]
- Yang, J.W.; Yoon, S.Y.; Oh, S.J.; Kim, S.K.; Kang, K.W. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2006, 346, 345–350. [Google Scholar] [CrossRef]
- Chen, L.M.; Tseng, H.Y.; Chen, Y.A.; Al Haq, A.T.; Hwang, P.A.; Hsu, H.L. Oligo-Fucoidan Prevents M2 Macrophage Differentiation and HCT116 Tumor Progression. Cancers 2020, 12, 421. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Song, Y.; Wang, Q.; Hu, Y.; He, Y.; Ren, D.; Wu, L.; Liu, S.; Cong, H.; Zhou, H. In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int. J. Biol. Macromol. 2019, 127, 48–56. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Surayot, U.; Lee, S.; You, S. Effects of sulfated fucan from the sea cucumber Stichopus japonicus on natural killer cell activation and cytotoxicity. Int. J. Biol. Macromol. 2018, 108, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, Z.; Wang, X.; Zhang, C.; Jiang, X. Natural killer cell awakening: Unleash cancer-immunity cycle against glioblastoma. Cell Death Dis. 2022, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Okimura, T.; Oda, T.; Jin, J.-O. Ascophyllan Induces Activation of Natural Killer Cells in Mice In Vivo and In Vitro. Mar. Drugs 2019, 17, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, E.K.; Hwang, J.; Kim, S.J.; Park, H.B.; Zhang, W.; Ryu, J.H.; You, S.; Jin, J.O. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. Int. J. Biol. Macromol. 2022, 208, 230–242. [Google Scholar] [CrossRef]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S.; et al. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef]
- Shukla, S.; Steinmetz, N.F. Emerging nanotechnologies for cancer immunotherapy. Exp. Biol. Med. (Maywood) 2016, 241, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.A.; Lin, X.Z.; Kuo, K.L.; Hsu, F.Y. Fabrication and Cytotoxicity of Fucoidan-Cisplatin Nanoparticles for Macrophage and Tumor Cells. Materials 2017, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.H.; Lu, K.Y.; Lee, W.C.; Hsu, W.J.; Lee, W.F.; Dai, J.Z.; Shueng, P.W.; Lin, C.W.; Mi, F.L. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials 2020, 257, 120227. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Choi, D.S.; Choi, S.; Won, J.Y.; Jo, Y.; Kim, H.B.; Jung, Y.; Shin, S.C.; Min, H.; Choi, H.W.; et al. Enhancing adoptive T-cell therapy with fucoidan-based IL-2 delivery microcapsules. Bioeng. Transl. Med. 2023, 8, e10362. [Google Scholar] [CrossRef]
- Moghbeli, M.; Khedmatgozar, H.; Yadegari, M.; Avan, A.; Ferns, G.A.; Ghayour Mobarhan, M. Chapter Five—Cytokines and the immune response in obesity-related disorders. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 101, pp. 135–168. [Google Scholar]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef]
- Ekmekcioglu, S.; Grimm, E.A.; Roszik, J. Targeting iNOS to increase efficacy of immunotherapies. Hum. Vaccin Immunother. 2017, 13, 1105–1108. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K.; Mori, N. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int. J. Oncol. 2012, 40, 251–260. [Google Scholar] [CrossRef]
- Jiang, S.; Yin, H.; Li, R.; Shi, W.; Mou, J.; Yang, J. The activation effects of fucoidan from sea cucumber Stichopus chloronotus on RAW264.7 cells via TLR2/4-NF-κB pathway and its structure-activity relationship. Carbohydr. Polym. 2021, 270, 118353. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1589. [Google Scholar] [CrossRef]
- Yadav, D.; Kwak, M.; Chauhan, P.S.; Puranik, N.; Lee, P.C.W.; Jin, J.-O. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin. Cancer Biol. 2022, 86, 909–922. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Kaveh, V.; Sheikholeslami, S.A.; Salari, S.; Bashash, D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit. Rev. Oncol. Hematol. 2021, 157, 103160. [Google Scholar] [CrossRef]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef]
- Tsai, H.L.; Tai, C.J.; Huang, C.W.; Chang, F.R.; Wang, J.Y. Efficacy of Low-Molecular-Weight Fucoidan as a Supplemental Therapy in Metastatic Colorectal Cancer Patients: A Double-Blind Randomized Controlled Trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2022, 25, 2167–2180. [Google Scholar] [CrossRef]


| Fucoidan Sources | Immune Cells | Involved Cytokines | Research Methods | Effects of Fucoidan | Ref. |
|---|---|---|---|---|---|
| Fucus vesiculosus | CD4+ T cells, CD8+ T cells, dendritic cells | IFN-γ, TNF-α, IL-12, IL-6, IL-12p40 | In vivo | Upregulated the production of IFN-γ and TNF-α in the presence of Th1 and Tc1 cells, to promotes CD8+ and CD4+ T cell responses. Induced maturation of DCs by upregulating TNF-α, IL-6, and IL-12p40 in spleen DCs. Increased the cell proliferation of CD44+ CD4 and memory T cells. Acts as an adjuvant to boost T cell immune responses. | [94] |
| Fucus Vesiculosus, Ascophyllum nodosum | CD4+ T cells, CD8+ T cells | IFN-γ, TNF-α, IL-3, IL-6, IL13, IL-14, L-24a, CSF2, CD70 | In vivo, In vitro | Promoted T cell proliferation and activation through upregulating IFN-γ and TNF-α secretion in CD8+ T cell populations. Promoted T cell proliferation via the JAK/STAT pathway. Interacted with TCR/CD3 complexes to enhance T cell activation. Co-administration with PD-1 antibody reduced tumor size and weight in mice. Co-administration with PD-1 antibody increased the ratio of CD8+ and CD4+ T cells in the spleen. | [18] |
| Fucus vesiculosus | CD8+ T cells, dendritic cells | IFN-γ | In vivo | Increased the cell proliferation of CD8+ T cells and also upregulated the production of IFN-γ. Inhibited SR-A in DCs by increasing the binding of NY-ESO-1 to DCs. In co-culture fucoidan-treated DCs with CD8+ T cells, serum IFN-γ increased. | [93] |
| Ecklonia cava | CD8+ T cells, CD4+ T cells | IFN-γ, TNF-α | In vivo | Activated T cells and enhanced T cell proliferations by increasing serum IFN-γ and TNF-α. Co-administration with PD-1 antibody prolonged survival in metastatic lung cancer. Acted as an adjuvant to enhance the therapeutic efficacy of immunotherapy. | [23] |
| Undaria pinnatifida | CD8+ T cells, CD4+ T cells, dendritic cells, CD11b+ macrophages, CD3-CD19-CD49b+ NK cells | TNF-α, IL-12, IL-6, TLR4, CD40, CD86, MHC I, MHC II, ERK, JNK, p38, p-iκB, p-NF-κB p65 | In vivo, In vitro | The LMWF-treated DCs activated T cells. The LMWF-treated DCs significantly increased CD4+ and CD8+ T cell proliferation. Activated the maturation of DCs by upregulating TLR4, CD40, CD86, MHC I, and MHC II. Activated the maturation of DCs by activating the TLR4, MAPK, and NF-κB signaling pathways. Activated the TLR4 signaling pathway by upregulating the phosphorylation of ERK, JNK, p38, and p-iκB while downregulating the level of p-NF-κB p65. Enhanced CD11b+ macrophage and CD3-CD19-CD49b+ NK cell proliferation by upregulating IL-6. Restored CTX-induced immunosuppression. | [35] |
| Fucus vesiculosus | Dendritic cells | IL-12, NF-α, FN-γ | In vitro | Activated the maturation of DCs by upregulating TNF-α, IFN-γ, and IL-12. | [131] |
| Laminaria Japonica, Laminaria cichoriodes, Fucus evanescens | HEK293 (human embryonic kidney cells) | NF-κB, TLR2, TLR4 | In vitro | Activated the NF-κB by interacting with human TLR2 and TLR4. | [134] |
| Fucus vesiculosus, Undariia pinnatifidat, Macrocystis pyrifera | Human peripheral blood mononuclear cells (PBMCs) | IFN-γ | In vitro | Promoted activation and proliferation of PBMCs. Reached the highest PBMC activation by increasing maximum IFN-γ secretion. Increased IFN-γ secretion after co-culture of Nivolumab-treated PBMCs and PC3 cells in the presence of anti-CD3. Fucoidan reached the highest activation level of PBMCs through increasing maximum IFN-γ secretion at the concentration of 10 μg/mL and 50 μg/mL. Inhibited PC3 proliferation. | [136] |
| Fucus evanescens | Human peripheral blood dendritic cells | TNF-α | In vivo | Induced PBDC maturation and increased TNF-α. | [132] |
| Fucus vesiculosus | M2 macrophages | TNF-α, IL-6, CCL22 | In vitro | Inhibited TNF-α and IL-6. Downregulated CCL22 chemokine by inhibiting p65-NF-κB phosphorylation. | [133] |
| Undaria pinnatifida | RAW 264.7 macrophages | TNF-α, IL-6, NO, iNOS, p38, κB-α, p65 | In vitro | Increased the expression of IL-6 and TNF-α. Enhanced NO and iNOS production. Activated the NF-κB signaling pathway by upregulating the phosphorylation levels of lκB-α and p65. Activated the MAPK signaling pathway by increasing p38 phosphorylation. | [95] |
| Unknown | RAW 264.7 macrophages | iNOS, NO, p38, SR-A | In vitro | Activated iNOS and increased NO production through the NF-κB and MAPK signaling pathways. | [137] |
| Undaria pinnatifida sporophyllus, Fucus vesiculosus | Spleen cells, B lymphocytes | IFN-γ, NO, CD25, CD69 | In vitro | Enhanced spleen cell proliferation and viability. Increased the expression of CD19, IFN-γ and NO on spleen cells. Increased a higher expression level of CD25 and CD69 on B lymphocytes. Increased the level of CD25 and CD69 on B lymphocytes. Increased spleen cell proliferation and viability. Reduced necrotic spleen cell populations. | [138] |
| Source of Fucoidan | Brief Description | Dosage Forms | Effects of Fucoidan | Ref. |
|---|---|---|---|---|
| Fucus vesiculosus | Combined fucoidan-based magnetic nanoparticles and immunomodulators enhance tumor-localized immunotherapy. | Nanoparticles (fucoidan-containing formulations: IO@FuDex1, IO@FuDex2, IO@FuDex3, M-IO@ FuDex1, M-IO@ FuDex3, and M-IO@ FuDex3) | IO@Fu-H: improved targeting efficiency; IO@Fu and IO@Fu: increased the cell association via a slow elevation of median fluorescence index (MFI) in 4T1 cells; IO@Fu-H: significantly increased the MFI in T cells; targeted PD-L1 receptors and associated with 4T1 cells; inhibited lung metastasis in 4T1 cancer model; M-IOFuDex (magnetic navigation): enhanced tumor selectivity; increased T cell proliferation; decreased Tregs and TAMs in TME; IO@FuDex and IO@Fu: inhibited the CT-26 tumor cell growth and extended the median survival to 62 days; reduced spleen Tregs; IO@Fu and M-IO@Fu increased TNF-, VEGF, and TGF- IO@Fu, IO@Fu, IO@Fu, and M-IO@Fu antitumoral effects and median survival. | [126] |
| Fucus vesiculosus | Enhanced adoptive T cell therapy using fucoidan-based IL-2 delivery microcapsules. | Microcapsules (fucoidan-based coacervate-laden injectable hydrogel ()) | Acted as an IL-2 delivery vehicle for enhancing adoptive T cell therapy (ACT); increased tumor-infiltrating T cells in CT26-bearing mice with injection than injection; Downregulated CD62L and enriched and cell generation; promoted STAT5 phosphorylation in T cells; increased Treg, NK, DNT, NKT, B, , and T cell populations; induced a higher Ki-67 expression in CT26-bearing mice; combination therapy (anti-PD-1 + ) reduced CT26 tumor cell growth and increased the IFN- levels in tumor-infiltrating T cells; increased naïve OT-I T and NY-ESO-1 TCR- T cell proliferation; decreased the expression of PD-1, Tim-3, TIGIT, and LAG-3 in tumor-infiltrating NY-ESO-1 TCR T cells. | [154] |
| Fucus vesiculosus | Cytotoxicity and fabrication of fucoidan-cisplatin nanoparticles for macrophage and tumor cells. | Nanoparticles | Increased the cell viability of RAW264.7 macrophages; non-cytotoxic to RAW264.7 macrophages; reduced the cytotoxicity of cisplatin; inhibited HCT-8 cell growth. | [152] |
| Laminaria japonica | Fucoidan-based and tumor-activated nanoplatform overcame hypoxia and enhanced photodynamic therapy and antitumor immunity. | Nanoparticles | Significantly increased the VP fluorescent emission in FM@VP-treated MDA-MB-231 cells; MDA-MB-231 cells took up greater FM@VP nanoparticle clusters; inhibited MDA-MB-231 and MDA-MB-468 cell growth; decreased TNBC cell viability, upregulated P-selectin level; overcome tumor hypoxia; decreased pro-angiogenesis generated by hypoxic tumor-elicited pro-angiogenesis; inhibited YAP levels, CTGF, cyclin D1, and EGFR in MDA-MB-231 cells; attenuated the Hippo signaling; downregulated the protein levels of PD-L1; enhanced T cell-mediated cytotoxicity; suppressed orthotopic 4T1 tumor cells growth and metastatic colonization of lung tumor; downregulated Treg cell infiltration; increased the expressions of granzyme B and IFN-; increased CD4 and CD8 T cells but decreased TAMs. | [153] |
| Cladosiphon okamuranus | Immunomodulatory effects of fucoidan in mice. | Oral gavage | Increased the proliferation of splenocytes that activated by concanavalin A and LPS, and increased macrophage phagocytosis activity and the levels of IL-2, IFN- serum IgM; decreased the levels of IL-4, IL-5 and serum IgE. | [2] |
| Fucus vesiculosus, Ascophyllu nodosum | Fucoidan-supplemented diet coordinated with ICBs to potentiate its antitumor immunity | Oral | Enhanced the therapeutic efficacy of PD-1 blockade; reduced B16 melanoma cell growth, volumes, and weights. Increased the proliferation of T, NK, and tumor-infiltrating T cells; activated DC maturation; increased the proliferation of T cells via increasing the production of IFN- and TNF- activated T cells through the JAK/STAT pathway. | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; McGowan, E.; Chen, S.; Santos, J.; Yin, H.; Lin, Y. Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy. Mar. Drugs 2023, 21, 128. https://doi.org/10.3390/md21020128
Li Y, McGowan E, Chen S, Santos J, Yin H, Lin Y. Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy. Marine Drugs. 2023; 21(2):128. https://doi.org/10.3390/md21020128
Chicago/Turabian StyleLi, Yani, Eileen McGowan, Size Chen, Jerran Santos, Haibin Yin, and Yiguang Lin. 2023. "Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy" Marine Drugs 21, no. 2: 128. https://doi.org/10.3390/md21020128