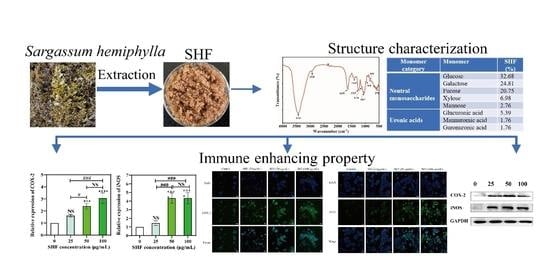
Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yields and Chemical Analysis of Fucoidan Obtained from Sargassum hemiphyllum
2.2. Molecular Properties
2.3. Monosaccharide Composition
2.4. FT-IR Analysis
2.5. Cellular Nitric Oxide Production
2.6. SHF Activates mRNA Expression of COX-2 and iNOS in Macrophages
2.7. Immunofluorescence
2.8. Western Blot
3. Materials and Methods
3.1. Reagents
3.2. Collection of Seaweed
3.3. Extraction and Purification of Sargassum Fucoidan
3.4. Yields and Chemical Compositions of Fucoidans
3.5. Determination of Molecular Properties
3.6. Monosaccharide Composition Analysis
3.7. FTIR Characterization
3.8. Cell line and Cell Culture
3.9. Determination of Nitric Oxide Production
3.10. Quantitative Real-Time PCR (qRT-PCR Analysis)
3.11. Immunofluorescence Staining
3.12. Western Blot Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Huang, M.; Cai, X.; Jia, L.; Wang, S. Investigation on activation in RAW264.7 macrophage cells and protection in cyclophosphamide-treated mice of Pseudostellaria heterophylla protein hydrolysate. Food Chem. Toxicol. 2019, 134, 110816. [Google Scholar] [CrossRef] [PubMed]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Immunomodulatory properties of photopolymerizable fucoidan and carrageenans. Carbohydr. Polym. 2020, 230, 115691. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, S.; Verzola, D.; Cappadona, F.; Bonino, B.; Murugavel, A.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell. Physiol. 2019, 234, 10868–10876. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Q.; Wang, J.; Wei, F.; Yang, L.; Ren, X. A new perspective: Exploring future therapeutic strategies for cancer by understanding the dual role of B lymphocytes in tumor immunity. Int. J. Cancer 2019, 144, 2909–2917. [Google Scholar] [CrossRef]
- Xin, G.L.L.K.; Khee, Y.P.; Ying, T.Y.; Chellian, J.; Gupta, G.; Kunnath, A.P.; Nammi, S.; Collet, T.; Hansbro, P.M.; Dua, K.; et al. Current status on immunological therapies for type 1 diabetes mellitus. Curr. Diab. Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, J.; Wu, L.; Yu, Y.; Richard, D.Y.; Zhang, Y.; Liang, X. In vitro immunomodulatory effects of human milk oligosaccharides on murine macrophage RAW264.7 cells. Carbohydr. Polym. 2019, 207, 230–238. [Google Scholar] [CrossRef]
- Christian, C.R.M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef]
- Rawat, C.; Kukal, S.; Dahiya, U.R.; Kukreti, R. Cyclooxygenase-2 (COX-2) inhibitors: Future therapeutic strategies for epilepsy management. J. Neuroinflammation 2019, 16, 197. [Google Scholar] [CrossRef]
- Ganesh, Y.; Gueorguieva, M.-S.; Lelutiu, J.; Dingledine, R. Cyclooxygenase-2 in epilepsy. Epilepsia J. Int. Leag. Against Epilepsy 2014, 55, 17–25. [Google Scholar]
- Salmeán, A.A.; Duffieux, D.; Harholt, J.; Qin, F.; Michel, G.; Czjzek, M.; Willats, W.G.T.; Hervé, C. Insoluble (1→3), (1→4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Sci. Rep. 2017, 7, 2880. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2002, 37, 175–223. [Google Scholar]
- Wang, G.; Sun, J.; Liu, G.; Wang, L.; Yu, J.; Liu, T.; Chi, S.; Liu, C.; Guo, H.; Wang, X.; et al. Comparative analysis on transcriptome sequencings of six Sargassum species in China. Acta Oceanol. Sin. 2014, 33, 37–44. [Google Scholar] [CrossRef]
- Yao, W.; Qiu, H.M.; Cheong, K.L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Bio.l Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, II; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal. Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Zhang, W.; Okimura, T.; Oda, T.; Jin, J.O. Ascophyllan induces activation of natural killer cells in mice in vivo and in vitro. Mar. Drugs 2019, 17, 197. [Google Scholar] [CrossRef] [Green Version]
- Leung, M.; Liu, C.; Koon, J.; Fung, K.P. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, Y.; Zhang, L.; Cheung, P.C.K.; Kennedy, J.F. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydr. Polym. 2007, 70, 324–333. [Google Scholar] [CrossRef]
- Hwang, P.A.; Wu, C.H.; Gau, S.Y.; Chien, S.Y.; Hwang, D.F. Antioxidant and immune-stimulating activities of hot-water extract from seaweed Sargassum Hemiphyllum. J. Mar. Sci. Technol. 2010, 18, 41–46. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Chien, S.-Y.; Chan, Y.-L.; Lu, M.-K.; Wu, C.-H.; Kong, Z.-L.; Wu, C.-J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Dabaghian, E.H.; You, S.; Yelithao, K.; Cao, R.; Rezaei, M.; Alboofetileh, M.; Bita, S. The activation of NF-κB and MAPKs signaling pathways of RAW264.7 murine macrophages and natural killer cells by fucoidan from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2020, 148, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.Y.; Chen, L.; Li, Q.J.; Shen, Y.Z.; Jin, L.; Zhang, X.; Chen, P.C.; Wu, M.J.; Choi, J.I.; et al. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Banu, A.T.; Ramani, P.S.; Murugan, A. Effect of seaweed coating on quality characteristics and shelf life of tomato (Lycopersicon esculentum mill). Food Sci. Hum. Wellness 2020, 9, 176–183. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Tai, M.R.C.; H., Y.; Li, R.; Jia, X.J.; Liu, X.F.; Ji, H.W.; Zhong, S.Y. Physicochemical properties and immunomodulatory effects of fucoidan from different brown algae. J. Guangdong Ocean. Univ. 2022, 42, 62–71. [Google Scholar]
- Terrell, E.; Dellon, L.D.; Dufour, A.; Bartolomei, E.; Broadbelt, L.J.; Garcia-Perez, M. A Review on Lignin Liquefaction: Advanced Characterization of Structure and Microkinetic Modeling. Ind. Eng. Chem. Res. 2019, 59, 526–555. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Dobrincic, A.; Pedisic, S.; Zoric, Z.; Jurin, M.; Roje, M.; Coz-Rakovac, R.; Dragovic-Uzelac, V. Microwave Assisted Extraction and Pressurized Liquid Extraction of Sulfated Polysaccharides from Fucus virsoides and Cystoseira barbata. Foods 2021, 10, 1481. [Google Scholar] [CrossRef]
- Khalafu, S.H.S.; Wan Aida, W.M.; Lim, S.J.; Maskat, M.Y. Effects of deodorisation methods on volatile compounds, chemical properties and antioxidant activities of fucoidan isolated from brown seaweed (Sargassum sp.). Algal Res. 2017, 25, 507–515. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical structure and molecular weight influence the in vitro fermentability of polysaccharide extracts from the edible seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Kim, W.J.; Koo, Y.K.; Jung, M.K.; Moon, H.R.; Kim, S.M.; Synytsya, A.; Yun-Choi, H.S.; Kim, Y.S.; Park, J.K.; Park, Y.I. Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Arch. Pharm. Res. 2010, 33, 125–131. [Google Scholar] [CrossRef]
- Long, H.; Gu, X.; Zhou, N.; Zhu, Z.; Wang, C.; Liu, X.; Zhao, M. Physicochemical characterization and bile acid-binding capacity of water-extract polysaccharides fractionated by stepwise ethanol precipitation from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 150, 654–661. [Google Scholar] [CrossRef]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular mechanisms of nonspecific immunity to intracellular infection: Cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Han, Y.; Yang, L.; Hu, L.; Duan, X.; Yang, X.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macromol. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.L.; Teng, B. Immunostimulatory effects of polysaccharides from Spirulina platensis in vivo and vitro and their activation mechanism on RAW246.7 macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Kim, A.J.; Kim, Y.O.; Hwang, J.K. Immunomodulating activity of Arabinogalactan and Focoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, S.H.; Ahn, G.; Cha, S.H.; Kim, A.D.; Yang, X.D.; Kang, M.C.; Jeon], Y.J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar]
- Song, K.-M.; Ha, S.J.; Lee, J.-E.; Kim, S.-H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT Food Sci. Technol. 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune Activation of RAW264.7 Macrophages by Low Molecular Weight Fucoidan Extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Guan, X.; Wang, Q.; Lin, B.; Sun, M.; Zheng, Q.; Huang, J.; Lai, G. Structural characterization of a soluble polysaccharide SSPS1 from soy whey and its immunoregulatory activity in macrophages. Int. J. Biol. Macromol. 2022, 217, 131–141. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Zhu, Y.; Li, Y.; Mei, S.; Luo, H.; Wu, K. Structural characterization of a mannoglucan polysaccharide from Dendrobium huoshanense and evaluation of its osteogenesis promotion activities. Int J Biol Macromol 2022, 211, 441–449. [Google Scholar] [CrossRef]
- Xia, X.; Hao, H.; Zhang, X.; Wong, I.N.; Chung, S.K.; Chen, Z.; Xu, B.; Huang, R. Immunomodulatory sulfated polysaccharides from Caulerpa racemosa var. peltata induces metabolic shifts in NF-κB signaling pathway in RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 182, 321–332. [Google Scholar] [CrossRef]






| Composition | Content (%) |
|---|---|
| Yield Rate | 2.72 ± 0.18 |
| Extraction Rate | 16.37 ± 1.96 |
| Total Polysaccharide | 75.35 ± 1.46 |
| Total Protein | 2.66 ± 0.67 |
| Total Polyphenol | 0.49 ± 0.01 |
| Sulfate | 44.11 ± 0.01 |
| Sample | Retention Time (min) | Relative Molecular Weight (kDa) | Relative Percentage of Peak Area (%) | ||
|---|---|---|---|---|---|
| Mw | Mn | Mp | |||
| SHF | 29.15 | 3374.86 | 1687.89 | 2097.88 | 7.68 |
| 31.65 | 1166.48 | 628.33 | 773.35 | 44.06 | |
| 36.44 | 152.37 | 94.61 | 114.29 | 14.50 | |
| 36.96 | 121.93 | 76.90 | 92.70 | 13.43 | |
| 39.31 | 44.94 | 30.39 | 36.30 | 5.91 | |
| 40.95 | 22.40 | 15.90 | 18.87 | 14.42 | |
| Monomer Category | Monomer | SHF |
|---|---|---|
| Neutral Monosaccharides | Glucose | 32.68 |
| Galactose | 24.81 | |
| Fucose | 20.75 | |
| Xylose | 6.98 | |
| Mannose | 2.76 | |
| Rhamnose | 1.03 | |
| Glucosamine Hydrochloride | 1.83 | |
| Galactose Hydrochloride | 0.24 | |
| Uronic Acids | Glucuronic Acid | 5.39 |
| Mannuronic Acid | 1.76 | |
| Guronuronic Acid | 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhou, Q.-L.; Chen, S.-T.; Tai, M.-R.; Cai, H.-Y.; Ding, R.; Liu, X.-F.; Chen, J.-P.; Luo, L.-X.; Zhong, S.-Y. Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum. Mar. Drugs 2023, 21, 18. https://doi.org/10.3390/md21010018
Li R, Zhou Q-L, Chen S-T, Tai M-R, Cai H-Y, Ding R, Liu X-F, Chen J-P, Luo L-X, Zhong S-Y. Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum. Marine Drugs. 2023; 21(1):18. https://doi.org/10.3390/md21010018
Chicago/Turabian StyleLi, Rui, Qing-Ling Zhou, Shu-Tong Chen, Min-Rui Tai, Hong-Ying Cai, Rui Ding, Xiao-Fei Liu, Jian-Ping Chen, Lian-Xiang Luo, and Sai-Yi Zhong. 2023. "Chemical Characterization and Immunomodulatory Activity of Fucoidan from Sargassum hemiphyllum" Marine Drugs 21, no. 1: 18. https://doi.org/10.3390/md21010018