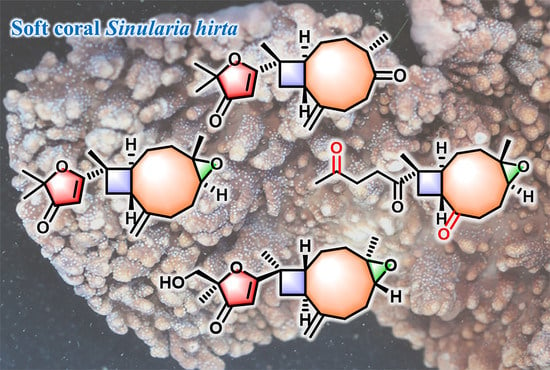
Sinuhirtone A, An Uncommon 17,19-Dinorxeniaphyllanoid, and Nine Related New Terpenoids from the Hainan Soft Coral Sinularia hirta
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds
3.5. QM-NMR Calculational Section
3.6. TDDFT-ECD Calculational Section
3.7. Optical Rotation Calculational Section
3.8. Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Pattenden, G. Novel macrocyclic and polycyclic norcembranoid diterpenes from Sinularia species of soft coral. Structural relationships and biosynthetic speculations. Nat. Prod. Rep. 2011, 28, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.U.; Liu, J.; Leng, X.; Ouyang, H. Chemical diversity and biological activity of secondary metabolites from soft coral genus Sinularia since 2013. Mar. Drugs 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, X.-W.; Li, H.; Yao, L.-G.; Tang, W.; Miao, Z.-H.; Wang, H.; Guo, Y.-W. Bioactive polyoxygenated cembranoids from a novel Hainan chemotype of the soft coral Sinularia flexibilis. Bioorg. Med. Chem. Lett. 2019, 29, 185–188. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Ru, T.; Yao, L.-G.; Miao, Z.-H.; Guo, Y.-W. Four new cembranoids from the Chinese soft coral Sinularia sp. and their anti-Aβ aggregation activities. Fitoterapia 2019, 136, 104176. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Zhang, Q.; Wu, Q.; Li, G.; Chen, K.-X.; Guo, Y.-W.; Tang, W.; Li, X.-W. Highly diverse cembranoids from the South China Sea soft coral Sinularia scabra as a new class of potential immunosuppressive agents. Bioorg. Med. Chem. 2019, 27, 3469–3476. [Google Scholar] [CrossRef]
- Liang, L.-F.; Wang, X.-J.; Zhang, H.-Y.; Liu, H.-L.; Li, J.; Lan, L.-F.; Zhang, W.; Guo, Y.-W. Bioactive polyhydroxylated steroids from the Hainan soft coral Sinularia depressa Tixier-Durivault. Bioorg. Med. Chem. Lett. 2013, 23, 1334–1337. [Google Scholar] [CrossRef]
- Chen, D.-W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.-H. Asteriscane-type sesquiterpenoids from the soft coral Sinularia capillosa. J. Nat. Prod. 2013, 76, 1753–1763. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, G.V.; Rao, N.S.K. Sesqui- and diterpenoids of the soft coral Sinularia hirta of the Andaman & Nicobar Islands. Indian J. Chem. B 1996, 35B, 815–818. [Google Scholar]
- Anjaneyulu, V.; Rao, K.N.; Babu, J.S.; Kobayashi, M. Isolation of 24-methylenecholesterol-3-O-α-L-fucopyranoside and 24-methylenecholest-7-ene-3β,6α,9α,11α-tetrol from a soft coral of the Andaman and Nicobar Islands. Indian J. Chem. B 1994, 33B, 144–147. [Google Scholar]
- Wu, Q.; Li, S.-W.; Xu, H.; Wang, H.; Hu, P.; Zhang, H.; Luo, C.; Chen, K.-X.; Nay, B.; Guo, Y.-W.; et al. Complex polypropionates from a South China Sea photosynthetic mollusk: Isolation and biomimetic synthesis highlighting novel rearrangements. Angew. Chem. Int. Ed. 2020, 59, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Li, W.-S.; Zhang, Z.-Y.; Luo, H.; Wang, J.-R.; Zhang, H.-Y.; Zeng, Z.-R.; Chen, B.; Li, X.-W.; Guo, Y.-W. Sinusiaetone A, an anti-inflammatory norditerpenoid with a bicyclo [11.3.0]hexadecane nucleus from the Hainan soft coral Sinularia siaesensis. Org. Lett. 2021, 23, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Tang, W.; Guo, Y.-W.; Li, X.-W. Klyflaccilides A and B, diterpenoids with 6/5/8/3 fused tetracyclic carbon skeleton from the Hainan soft coral Klyxum flaccidum. Org. Lett. 2019, 21, 5660–5664. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wu, Y.; Zhu, Z.-D.; Mollo, E.; Gavagnin, M.; Gu, Y.-C.; Zhu, W.-L.; Li, X.-W.; Guo, Y.-W. Sarinfacetamides A and B, nitrogenous diterpenoids with tricyclo[6.3.1.01,5]dodecane scaffold from the South China Sea soft coral Sarcophyton infundibuliforme. Org. Lett. 2018, 20, 2637–2640. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, Z.-D.; Chen, J.-S.; Li, J.; Gu, Y.-C.; Zhu, W.-L.; Li, X.-W.; Guo, Y.-W. Xishacorenes A–C, diterpenes with bicyclo[3.3.1]nonane nucleus from the Xisha soft coral Sinularia polydactyla. Org. Lett. 2017, 19, 4183–4186. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Q.; Li, X.-W.; Li, S.-W.; Cui, Z.; Guo, Y.-W.; Han, G.-Y. Sinuhirtins A and B, two uncommon norhumulene-type terpenoids from the South China Sea soft coral Sinularia hirta. Tetrahedron Lett. 2019, 60, 151308. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Su, J.-H.; Shiue, R.-T.; Pan, X.-J.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. New β-caryophyllene-derived terpenoids from the soft coral Sinularia nanolobata. J. Nat. Prod. 2004, 67, 592–597. [Google Scholar] [CrossRef]
- Chen, S.-P.; Chao, C.-H.; Huang, H.-C.; Wu, Y.-C.; Lu, C.-K.; Dai, C.-F.; Sheu, J.-H. New β-caryophyllene-derived terpenoids from the Formosan soft coral Sinularia gibberosa. Bull. Chem. Soc. Jpn. 2006, 79, 1547–1551. [Google Scholar] [CrossRef]
- Chen, S.-P.; Su, J.-H.; Yeh, H.-C.; Ahmed, A.F.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Novel norhumulene and xeniaphyllane-derived terpenoids from a Formosan soft coral Sinularia gibberosa. Chem. Pharm. Bull. 2009, 57, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.R.; Ernst, L.; Stumpf, B. Biotransformation of caryophyllene by Diplodia gossypina. Phytochemistry 1990, 29, 115–120. [Google Scholar] [CrossRef]
- Mennucci, B.; Claps, M.; Evidente, A.; Rosini, C. Absolute configuration of natural cyclohexene oxides by time dependent density functional theory calculation of the optical rotation: The absolute configuration of (−)-sphaeropsidone and (−)-episphaeropsidone revised. J. Org. Chem. 2007, 72, 6680–6691. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Xie, C.-L.; Xia, J.-M.; Liu, Q.-M.; Peng, G.-Z.; Liu, G.-M.; Yang, X.-W.; Botryotins, A.-H. Tetracyclic diterpenoids representing three carbon skeletons from a deep-sea-derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Yao, L.-G.; Wu, Q.; Guo, Y.-W. Uncommon polycyclic merosesquiterpenoids and asteriscanoids from the Hainan soft coral Sinularia humesi. Chin. J. Chem. 2021, 39, 2377–2385. [Google Scholar] [CrossRef]
- Sun, L.-L.; Li, W.-S.; Li, J.; Zhang, H.-Y.; Yao, L.-G.; Luo, H.; Guo, Y.-W.; Li, X.-W. Uncommon diterpenoids from the South China Sea soft coral Sinularia humilis and their stereochemistry. J. Org. Chem. 2021, 86, 3367–3376. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]









| No. | 1 a | 2 a | 3 a | 4 a | 5 a | 6 a |
|---|---|---|---|---|---|---|
| δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | |
| 1 | 2.45 t (9.6) | 2.09 m | 2.48 t (9.6) | 1.89 t (9.6) | 1.90 t (7.8) | 2.12 m |
| 2a | 1.76 dt (14.4, 3.6) | 1.66 m | 1.77 m | 1.64 m | 1.51 m | 1.73 m |
| 2b | 1.60 m | 1.37 m | 1.60 m | 1.43 m | 1.24 m | 1.37 m |
| 3a | 2.12 dt (13.2, 3.6) | 1.84 m | 2.10 m | 2.06 dt (13.2, 3.6) | 1.88 m | 1.92 m |
| 3b | 0.97 td (13.2, 5.4) | 1.62 m | 0.98 td (13.2, 4.8) | 0.96 td (13.2, 4.8) | 1.68 m | 1.67 m |
| 4 | 2.52 m | 2.52 m | 2.54 m | |||
| 5 | 2.85 dd (10.8, 4.2) | 2.86 dd (10.2, 4.2) | 2.88 dd (10.2, 4.2) | |||
| 6a | 2.16 ddd (13.2, 7.8, 4.8) | 2.51 m | 2.27 m | 2.13 ddd (15.0, 7.8, 4.2) | 2.53 m | 2.53 m |
| 6b | 1.34 overlap | 1.32 m | 1.32 m | |||
| 7a | 2.35 m | 2.49 m | 2.37 ddd (13.2, 8.4, 4.2) | 2.33 ddd (13.2, 8.4, 4.2) | 2.45 m | 2.51 m |
| 7b | 2.17 m | 2.17 m | 2.25 ddt (12.0, 7.8, 4.2) | 2.48 m | ||
| 9 | 2.79 q (9.6) | 2.56 m | 2.81 q (9.6) | 2.64 q (9.6) | 2.50 m | 2.53 m |
| 10a | 2.26 m | 2.16 t (10.8) | 2.29 m | 1.65 m | 1.61 m | 2.06 t (10.2) |
| 10b | 1.87 dd (10.8, 8.4) | 1.80 dd (10.8, 8.4) | 1.89 dd (10.8,8.4) | 1.52 m | 1.77 dd (11.4, 8.4) | |
| 12 | 2.00 d (6.6) | 1.91 m | ||||
| 13 | 5.30 s | 5.26 s | 5.37 s | 5.59 overlap | 5.55 ddd (15.6, 7.8, 6.6) | 6.47 d (15.0) |
| 14 | 5.61 overlap | 5.61 d (15.6) | 6.92 d (15.0) | |||
| 16a | 1.37 s | 1.38 s | 3.81 d (12.0) | 1.30 s | 1.33 s | 1.40 s |
| 16b | 3.69 d (12.0) | |||||
| 17 | 1.37 s | 1.37 s | 1.37 s | 1.30 s | 1.39 s | 1.40 s |
| 18 | 1.36 s | 1.29 s | 1.39 s | 1.03 s | 0.98 s | 1.21 s |
| 19a | 5.07 s | 4.96 s | 5.07 s | 4.97 s | 4.86 s | 4.91 s |
| 19b | 4.96 s | 4.95 s | 4.96 s | 4.86 s | 4.82 s | 4.91 s |
| 20 | 1.22 s | 1.02 s | 1.21 s | 1.19 s | 1.00 d (6.6) | 1.03 d (6.6) |
| No. | 1 a | 2 a | 3 a | 4 a | 5 a | 6 a | 7 a | 8 a | 9 a | 10 a |
|---|---|---|---|---|---|---|---|---|---|---|
| δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | δC Mult. | |
| 1 | 48.4 CH | 49.0 CH | 48.7 CH | 48.8 CH | 44.6 CH | 45.7 CH | 45.8 CH | 45.5 CH | 58.1 CH | 52.7 CH |
| 2 | 27.7 CH2 | 27.1 CH2 | 27.7 CH2 | 27.9 CH2 | 26.2 CH2 | 27.0 CH2 | 27.4 CH2 | 28.3 CH2 | 29.8 CH2 | 26.1 CH2 |
| 3 | 38.9 CH2 | 30.2 CH2 | 38.8 CH2 | 39.0 CH2 | 29.2 CH2 | 29.5 CH2 | 38.5 CH2 | 38.7 CH2 | 32.5 CH2 | 39.0 CH2 |
| 4 | 59.5 qC | 48.2 CH | 59.5 qC | 59.9 qC | 47.2 CH | 48.1 CH | 58.9 qC | 59.5 qC | 150.9 qC | 59.8 qC |
| 5 | 63.8 CH | 216.6 qC | 63.7 CH | 64.0 CH | 218.4 qC | 217.5 qC | 61.8 CH | 63.6 CH | 75.7 CH | 63.4 CH |
| 6 | 30.1 CH2 | 41.8 CH2 | 30.1 CH2 | 30.3 CH2 | 43.4 CH2 | 41.9 CH2 | 25.0 CH2 | 30.1 CH2 | 32.3 CH2 | 29.9 CH2 |
| 7 | 29.8 CH2 | 32.3 CH2 | 29.8 CH2 | 29.9 CH2 | 31.4 CH2 | 32.1 CH2 | 37.6 CH2 | 29.9 CH2 | 33.2 CH2 | 30.8 CH2 |
| 8 | 150.5 qC | 151.9 qC | 150.4 qC | 151.9 qC | 153.3 qC | 152.2 qC | 213.3 qC | 150.5 qC | 151.1 qC | 151.4 qC |
| 9 | 48.1 CH | 42.7 CH | 48.1 CH | 48.8 CH | 44.0 CH | 42.2 CH | 51.3 CH | 47.4 CH | 40.0 CH | 48.2 CH |
| 10 | 35.7 CH2 | 35.0 CH2 | 35.6 CH2 | 38.1 CH2 | 35.7 CH2 | 34.4 CH2 | 31.4 CH2 | 35.2 CH2 | 40.3 CH2 | 40.5 CH2 |
| 11 | 39.0 qC | 38.9 qC | 39.2 qC | 37.3 qC | 38.1 qC | 47.7 qC | 48.2 qC | 48.2 qC | 70.7 qC | 73.6 qC |
| 12 | 196.2 qC | 196.2 qC | 198.1 qC | 46.3 CH2 | 44.3 CH2 | 203.8 qC | 212.5 qC | 203.2 qC | 21.7 CH3 | 28.3 CH3 |
| 13 | 98.6 CH | 98.5 CH | 100.4 CH | 123.1 CH | 123.4 CH | 120.7 CH | 30.5 CH2 | 132.7 CH | ||
| 14 | 207.6 qC | 207.8 qC | 206.1 qC | 140.7 CH | 141.5 CH | 153.3 CH | 37.0 CH2 | 137.8 CH | 110.0 CH2 | 113.0 CH2 |
| 15 | 88.7 qC | 88.7 qC | 90.5 qC | 70.9 qC | 70.4 qC | 71.1 qC | 207.2 qC | 197.9 qC | 114.6 CH2 | 16.9 CH3 |
| 16 | 23.0 CH3 | 23.0 CH3 | 65.9 CH2 | 30.2 CH3 | 33.0 CH3 | 29.3 CH3 | 30.1 CH3 | 29.3 CH3 | ||
| 17 | 23.1 CH3 | 23.1 CH3 | 18.4 CH3 | 30.1 CH3 | 29.4 CH3 | 29.4 CH3 | ||||
| 18 | 17.4 CH3 | 17.4 CH3 | 17.4 CH3 | 19.8 CH3 | 21.0 CH3 | 16.6 CH3 | 17.6 CH3 | 16.7 CH3 | ||
| 19 | 114.5 CH2 | 112.9 CH2 | 114.6 CH2 | 113.1 CH2 | 112.0 CH2 | 112.7 CH2 | 114.4 CH2 | |||
| 20 | 17.1 CH3 | 16.2 CH3 | 17.1 CH3 | 17.2 CH3 | 15.2 CH3 | 15.6 CH3 | 16.4 CH3 | 17.2 CH3 |
| No. | 7 a | 8 b | 9 a | 10 a |
|---|---|---|---|---|
| δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | δH Mult (J Hz) | |
| 1 | 2.64 m | 2.47 t (9.5) | 1.86 m | 1.87 t (9.6) |
| 2a | 1.84 dt (15.0, 3.6) | 1.83 m | 1.97 m | 1.74 dt (14.4, 4.2) |
| 2b | 1.64 m | 1.59 m | 1.56 m | 1.65 m |
| 3a | 2.15 dt (13.2, 3.6) | 2.12 m | 2.57 dt (13.2, 4.2) | 2.16 dt (13.2, 3.6) |
| 3b | 1.09 td (13.2, 4.8) | 1.10 td (13.0, 5.0) | 1.95 m | 1.00 td (13.2, 4.8) |
| 5 | 2.76 dd (10.2, 4.2) | 2.88 dd (10.5, 4.5) | 4.12 dd (9.6, 3.6) | 2.83 dd (10.2, 4.2) |
| 6a | 2.40 m | 2.25 m | 1.95 m | 2.24 m |
| 6b | 1.46 m | 1.33 m | 1.79 m | 1.35 m |
| 7a | 2.57 m | 2.30 m | 2.35 ddd (14.4, 10.2, 4.2) | 2.35 m |
| 7b | 2.13 m | 2.09 m | 2.09 m | |
| 9 | 3.08 q (9.0) | 2.75 q (9.5) | 1.88 m | 2.88 q (9.6) |
| 10a | 2.63 m | 2.15 m | 2.00 m | 2.02 m |
| 10b | 1.79 dd (11.4, 7.8) | 1.90 dd (11.0, 8.0) | 1.93 m | |
| 12 | 1.24 s | 1.27 s | ||
| 13 | 2.65 m | 6.98 s | ||
| 14a | 2.73 m | 6.98 s | 4.83 s | 5.02 s |
| 14b | 4.79 s | 4.93 s | ||
| 15a | 5.05 s | 1.25 s | ||
| 15b | 4.96 s | |||
| 16 | 2.19 s | 2.36 s | ||
| 18 | 1.34 s | 1.34 s | ||
| 19a | 5.00 s | |||
| 19b | 4.93 s | |||
| 20 | 1.30 s | 1.22 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-H.; Lu, S.-Q.; Han, G.-Y.; Li, X.-W.; Guo, Y.-W. Sinuhirtone A, An Uncommon 17,19-Dinorxeniaphyllanoid, and Nine Related New Terpenoids from the Hainan Soft Coral Sinularia hirta. Mar. Drugs 2022, 20, 272. https://doi.org/10.3390/md20040272
Chen Z-H, Lu S-Q, Han G-Y, Li X-W, Guo Y-W. Sinuhirtone A, An Uncommon 17,19-Dinorxeniaphyllanoid, and Nine Related New Terpenoids from the Hainan Soft Coral Sinularia hirta. Marine Drugs. 2022; 20(4):272. https://doi.org/10.3390/md20040272
Chicago/Turabian StyleChen, Zi-Hui, Si-Qi Lu, Guan-Ying Han, Xu-Wen Li, and Yue-Wei Guo. 2022. "Sinuhirtone A, An Uncommon 17,19-Dinorxeniaphyllanoid, and Nine Related New Terpenoids from the Hainan Soft Coral Sinularia hirta" Marine Drugs 20, no. 4: 272. https://doi.org/10.3390/md20040272