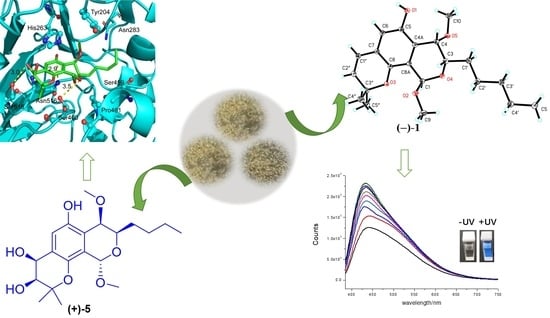
Pyranodipyran Derivatives with Tyrosyl DNA Phosphodiesterase 1 Inhibitory Activities and Fluorescent Properties from Aspergillus sp. EGF 15-0-3
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain and Fermentation
3.3. Extraction and Isolation
3.4. Structural Characterizations of 1–6
3.5. Chiral Separation of 1–6
3.6. Quantum Chemical Calculations
3.7. X-ray Crystallographic Analysis
3.8. TDP1 Inhibition Assay
3.9. Molecular Modeling
3.10. Measurement of Fluorescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Dexheimer, T.S.; Antony, S.; Marchand, C.; Pommier, Y. Tyrosyl-DNA Phosphodiesterase as a target for anticancertherapy. Anti-Cancer Agents Med. Chem. 2008, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, E.Q.; Waardenburg, R.C.A.M.V. Tyrosyl-DNA phosphodiesterase I resolves both naturally and chemically induced DNA adducts and its potential as a therapeutic target. Drug Metab. Rev. 2014, 46, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Interthal, H.; Pouliott, J.J.; Champoux, J.J. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc. Natl. Acad. Sci. USA 2001, 98, 12009–12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Saha, S.; Wang, W.; Saha, L.K.; Huang, S.N.; Pommier, Y. Excision repair of topoisomerase DNA-protein crosslinks (TOPDPC). DNA Repair. 2020, 89, 102837. [Google Scholar] [CrossRef] [PubMed]
- Nivens, M.C.; Felder, T.; Galloway, A.H.; Pena, M.M.; Pouliot, J.J.; Spencer, H.T. Engineered resistance to camptothecin and antifolates by retroviral coexpression of tyrosyl DNA phosphodiesterase-I and thymidylate synthase. Cancer Chemother. Pharmacol. 2004, 53, 107–115. [Google Scholar]
- Barthelmes, H.U.; Habermeyer, M.; Christensen, M.O.; Mielke, C.; Interthal, H.; Pouliot, J.J.; Boege, F.; Marko, D. TDP1 overexpression in human cells counteracts DNA damage mediated by topoisomerases I and II. J. Biol. Chem. 2004, 279, 55618–55625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharenko, A.; Luzina, O.; Koval, O.; Nilov, D.I.; Gushchina, N.; Dyrkheeva, V.; Svedas, N.; Salakhutdinov, O.L. Tyrosyl-DNA phosphodiesterase 1 inhibitors: Usnic acid enamines enhance the cytotoxic effect of camptothecin. J. Nat. Prod. 2016, 79, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Wang, H.W.; Tang, W.L.; Zhang, Y.; Yang, H.; Hu, D.X.; Ravji, A.; Marchand, C.; Kiselev, E.; Ofori-Atta, K.; et al. Discovery, Synthesis, and evaluation of oxynitidine derivatives as dual Inhibitors of DNA topoisomerase IB (TOP1) and tyrosyl-DNA phosphodiesterase 1 (TDP1), and potential antitumor agents. J. Med. Chem. 2018, 61, 9908–9930. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, K.; Oleshko, O.; Mamontova, E.; Yarovaya, O.; Zakharova, O.; Zakharenko, A.; Kononova, A.; Dyrkheeva, N.; Cheresiz, S.; Pokrovsky, A.; et al. Dehydroabietylamine ureas and thioureas as tyrosyl-DNA phosphodiesterase 1 inhibitors that enhance the antitumor effect of temozolomide on glioblastoma cells. J. Nat. Prod. 2019, 82, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Laev, S.S.; Salakhutdinov, N.F.; Lavrik, O.I. Tyrosyl-DNA phosphodiesterase inhibitors: Progress and potential. Bioorgan. Med. Chem. 2016, 24, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Bantick, J.R.; Cairns, H.; Chambers, A.; Hazard, R.; King, J.; Lee, T.B. Benzodipyran derivatives with antiallergic activity. J. Med. Chem. 1976, 19, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, N.Y.; Lin, H.C.; Lee, C.Y.; Hung, C.C.; Chang, C.S. Synthesis and bioevaluation of novel benzodipyranone derivatives as P-glycoprotein inhibitors for multidrug resistance reversal agents. Eur. J. Med. Chem. 2016, 118, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, C.L.; Wang, K.L.; Xu, Y.; She, Z.G.; Wang, C.Y. Dihydroisocoumarin derivatives with antifouling activities from a gorgonian-derived Eurotium sp. fungus. Tetrahedron 2014, 70, 9132–9138. [Google Scholar] [CrossRef]
- Xiao, L.G.; Zhang, Y.; Zhang, H.L.; Li, D.; Gu, Q.; Tang, G.H.; Yu, Q.; An, L.K. Spiroconyone A, a new phytosterol with a spiro [5,6] ring system from Conyza japonica. Org. Biomol. Chem. 2020, 18, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, Y.; Yan, X.L.; Xiao, L.G.; Hu, D.X.; Yu, Q.; An, L.K. Secondary metabolites from Isodon ternifolius (D. Don) Kudo and their anticancer activity as DNA topoisomerase IB and Tyrosyl-DNA phosphodiesterase 1 inhibitors. Bioorg. Med. Chem. 2020, 28, 115527. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, J.C.; Hu, J.S.; He, X.X.; Lin, S.J.; Zhang, D.M.; Ye, W.C.; Chen, M.F.; Lin, H.W.; Zhang, C.X. Probing indole diketopiperazine-based hybrids as environmental-induced products from Aspergillus sp. EGF 15-0-3. Org. Lett. 2022, 24, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Chen, N.N.; Li, J.; Su, J.C.; Yang, J.Y.; Zhang, C.X.; Lin, H.W.; Zhou, Y.J. Antimicrobial chlorinated carbazole alkaloids from the sponge-associated actinomycete Streptomyces diacarni LHW51701. Chin. J. Chem. 2021, 39, 1188–1192. [Google Scholar] [CrossRef]
- Liu, B.X.; Wei, X.; Xiao, X.J.; Zhang, Q.; Zhang, C.X. Research on benzaldehydes from the soft coral-associated symbiotic fungus Aspergillus sp. EGF15-0-3. J. Trop. Oceanogr. 2021, 40, 63–69. [Google Scholar]
- Nakayama, A.; Sato, H.; Nagano, S.J.; Karanjit, S.; Imagawa, H.; Namba, K. Asymmetric total syntheses and structure elucidations of (+)-eurotiumide F and (+)-eurotiumide G. Chem. Pharm. Bull. 2019, 67, 953–958. [Google Scholar] [CrossRef]
- Snatzke, G.; Wagner, U.; Wolff, H.P. Circulardichroism—LXXV1: Cottonogenic derivatives of chiral bidentate ligands with the complex [Mo2 (O2CCH3)4]. Tetrahedron 1981, 37, 349–361. [Google Scholar] [CrossRef]
- Górecki, M.; Jabłońska, E.; Kruszewska, A.; Suszczyńska, A.; Urbańczyk-Lipkowska, Z.; Gerards, M.; Morzycki, J.W.; Szczepek, W.J.; Frelek, J. Practical method for the absolute configuration assignment of tert/tert 1,2-diols using their complexes with Mo2(OAc)4. J. Org. Chem. 2007, 72, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Lountos, G.T.; Zhao, X.Z.; Evgeny, K.; Tropea, J.E.; Needle, D.; Pommier, Y.; Burke, T.R.; Waugh, D.S. Identification of a ligand binding hot spot and structural motifs replicating aspects of tyrosyl-DNAphosphodiesterase I (TDP1) phosphoryl recognitionby crystallographic fragment cocktail screening. Nucleic Acids Res. 2019, 19, 10134–10150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Feng, C.; Wang, S.Y.; Zhang, D.M.; Li, X.H.; Zhang, C.X. New indole diketopiperazine alkaloids from soft coral-associated epiphytic fungus Aspergillus sp. EGF 15-0-3. Chem. Biodive. 2020, 17, e2000106. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Song, J.G.; Su, J.C.; Song, Q.Y.; Huang, R.L.; Tang, W.; Hu, L.J.; Huang, X.J.; Jiang, R.W.; Li, Y.L.; Ye, W.C.; et al. Cleistocaltones A and B, Antiviral phloroglucinol−terpenoid adducts from Cleistocalyx operculatus. Org. Lett. 2019, 21, 9579–9583. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis Version 1.70; University of Wuerzburg: Wuerzburg, Germany, 2017. [Google Scholar]
- Su, J.C.; Wang, S.; Cheng, W.; Huang, X.J.; Li, M.M.; Jiang, R.W.; Li, Y.L.; Wang, L.; Ye, W.C.; Wang., Y. Phloroglucinol derivatives with unusual skeletons from Cleistocalyx operculatus and their in vitro antiviral activity. J. Org. Chem. 2018, 83, 8522–8532. [Google Scholar] [CrossRef]
- Hu, D.X.; Tang, W.L.; Zhang, Y.; Yang, H.; Wang, W.; Agama, K.; Pommier, Y.; An, L.K. Synthesis of methoxy-, methylenedioxy-, hydroxy-, and haloSubstituted benzophenanthridinone derivatives as DAN topoisomerase IB (TOP1) and tyrosyl-DNA phosphodiesterase 1 (TDP1) inhibitors and their biological activity for drug-resistant cancer. J. Med. Chem. 2021, 64, 7617–7629. [Google Scholar] [CrossRef]











| No. | 1 b | 2 b | 3 b | 4 b | 5 b | 6 c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH, mult, J | δC, mult | δH, mult, J | δC, mult | δH, mult, J | δC, mult | δH, mult, J | δC, mult | δH, mult, J | δC, mult | δH, mult, J | δC, mult | |
| 1 | 5.56, s | 95.4, CH | 5.42, s | 95.2, CH | 5.91, s | 95.0, CH | 5.51, s | 95.3, CH | 5.52, s | 95.2, CH | 5.55, s | 96.6, CH |
| 2 | ||||||||||||
| 3 | 4.20, dt (6.8, 3.2) | 70.2, CH | 4.20, dt (10.0, 3.0) | 64.8, CH | 4.26, dt (6.8, 3.2) | 70.8, CH | 4.18, dt (7.2, 3.2) | 70.1, CH | 4.18, dt (7.0, 3.2) | 70.5, CH | 4.00, ddd (7.2, 4.4, 1.6) | 72.6, CH |
| 4 | 4.44, d (3.2) | 68.8, CH | 4.63, d (10.0) | 76.0, CH | 4.56, d (3.2) | 68.9, CH | 4.44, d (3.2) | 68.7, CH | 4.43, d (3.2) | 68.9, CH | 4.34, d (1.6) | 70.9, CH |
| 4a | 117.8, C | 117.8, C | 114.9, C | 117.3, C | 116.5, C | 117.9, C | ||||||
| 5 | 149.1, C | 149.4, C | 151.3, C | 150.5, C | 150.2, C | 151.4, C | ||||||
| 6 | 6.58, s | 113.3, CH | 6.51, s | 113.4, CH | 7.05, s | 106.9, CH | 6.75, s | 112.8, CH | 6.76, s | 112.9, CH | 6.89, s | 112.9, CH |
| 7 | 122.5, C | 122.5, C | 129.4, C | 116.2, C | 117.4, C | 123.0, C | ||||||
| 8 | 143.4, C | 143.0, C | 146.3, C | 149.3, C | 149.6, C | 150.9, C | ||||||
| 8a | 124.1, C | 124.2, C | 119.9, C | 128.8, C | 128.8, C | 131.3, C | ||||||
| 1′ | 1.79, m | 30.4, CH2 | 1.78, m | 31.9, CH2 | 1.86, m | 30.5, CH2 | 1.65, m | 30.8, CH2 | 1.80, m | 30.4, CH2 | 1.30, m | 31.9, CH2 |
| 2′ | 1.66, m | 25.9, CH2 | 1.66, m | 25.2, CH2 | 1.41, m | 26.0, CH2 | 1.61, m | 26.0, CH2 | 1.25, m | 26.0, CH2 | 1.45, m | 26.8, CH2 |
| 3′ | 1.61, m | 32.0, CH2 | 1.62, m | 32.0, CH2 | 1.40, m | 32.0, CH2 | 1.39, m | 32.0, CH2 | 1.39, m | 32.0, CH2 | 1.40, m | 33.0, CH2 |
| 4′ | 1.51, m | 22.8, CH2 | 1.51, m | 22.8, CH2 | 1.40, m | 22.8, CH2 | 1.51, m | 30.4, CH2 | 1.33, m | 22.8, CH2 | 1.42, m | 23.7, CH2 |
| 5′ | 0.93, t (6.4) | 14.2, CH3 | 0.92, t (7.2) | 14.2, CH3 | 0.94, t (6.8) | 14.3, CH3 | 0.93, t (6.6) | 14.2, CH3 | 0.93, t (6.4) | 14.2, CH3 | 0.95, t (6.8) | 14.4, CH3 |
| 1″ | 6.28, d (10.0) | 122.2, CH | 6.28, d (9.6) | 122.1, CH | 6.57, s | 103.8, CH | 1.81, m | 22.8, CH2 | 3.18, m a3.14, d (8.4) | 31.0, CH2 | 5.31, d (4.8) | 74.0, CH |
| 2″ | 5.68, d (10.0) | 132.4, CH | 5.68, d (9.6) | 132.2, CH | 161.5, C | 4.74, dt (6.8, 3.2) | 88.4, CH | 4.69, t (8.4) | 89.7, CH | 4.20, t (4.8) | 98.3, CH | |
| 3″ | 77.4, C | 77.4, C | 73.6, C | 76.1, C | 72.1, C | 72.0, C | ||||||
| 4″ | 1.41, s | 27.4, CH3 | 1.41, s | 27.6, CH3 | 1.62, s | 25.9, CH3 | 1.24, s | 22.2, CH3 | 1.33, s | 24.3, CH3 | 1.27, s | 25.4, CH3 |
| 5″ | 1.41, s | 28.0, CH3 | 1.40, s | 28.0, CH3 | 1.61, s | 25.1, CH3 | 1.13, s | 19.6, CH3 | 1.19, s | 26.1, CH3 | 1.25, s | 25.8, CH3 |
| 1-OCH3 | 3.54, s | 55.8, CH3 | 3.53, s | 56.1, CH3 | 3.63, s | 55.9, CH3 | 3.53, s | 55.8, CH3 | 3.53, s | 55.6, CH3 | 3.45, s | 58.4, CH3 |
| 4-OCH3 | 3.18, s | 53.9, CH3 | 3.18, s | 51.4, CH3 | 3.20, s | 53.9, CH3 | 3.26, s | 53.8, CH3 | 3.19, s | 53.9, CH3 | 3.35, s | 49.6, CH3 |
| 2″-OCH3 | 3.15, s | 51.3, CH3 | 3.17, s | 50.2, CH3 | ||||||||
| Compound | TDP1 Inhibition (μM) | Compound | TDP1 Inhibition (μM) |
|---|---|---|---|
| (±)-1 | >100 | (±)-4 | >100 |
| (+)-1 | >100 | (+)-4 | >100 |
| (−)-1 | >100 | (−)-4 | >100 |
| (±)-2 | 57.81 ± 2.20 | (±)-5 | >100 |
| (+)-2 | >100 | (+)-5 | >100 |
| (−)-2 | >100 | (−)-5 | >100 |
| (±)-3 | >100 | (±)-6 | 6.50 ± 0.73 |
| (+)-3 | >100 | (+)-6 | 27.76 ± 1.73 |
| (−)-3 | >100 | (−)-6 | 37.31 ± 3.63 |
| Probes | λabs | λemb | Stokes Shift | ε [M–1cm–1] | φF |
|---|---|---|---|---|---|
| 1 | 221 | 445 | 224 | 125388.61 | 0.0392 |
| 2 | 223 | 464 | 241 | 70590.11 | 0.0225 |
| 3 | 206 | 472 | 266 | 61279.01 | 0.0048 |
| 4 | 225 | 428 | 203 | 208307.41 | 0.0649 |
| 5 | 203 | 432 | 229 | 106186.60 | 0.0482 |
| 6 | 225 | 424 | 199 | 127827.28 | 0.0416 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, F.-T.; Si-Tu, M.-X.; Fan, H.; Hu, J.-S.; Yang, H.; Guan, S.-Y.; An, L.-K.; Zhang, C.-X. Pyranodipyran Derivatives with Tyrosyl DNA Phosphodiesterase 1 Inhibitory Activities and Fluorescent Properties from Aspergillus sp. EGF 15-0-3. Mar. Drugs 2022, 20, 211. https://doi.org/10.3390/md20030211
Wei X, Wang F-T, Si-Tu M-X, Fan H, Hu J-S, Yang H, Guan S-Y, An L-K, Zhang C-X. Pyranodipyran Derivatives with Tyrosyl DNA Phosphodiesterase 1 Inhibitory Activities and Fluorescent Properties from Aspergillus sp. EGF 15-0-3. Marine Drugs. 2022; 20(3):211. https://doi.org/10.3390/md20030211
Chicago/Turabian StyleWei, Xia, Fang-Ting Wang, Mei-Xia Si-Tu, Hao Fan, Jin-Shan Hu, Hao Yang, Shan-Yue Guan, Lin-Kun An, and Cui-Xian Zhang. 2022. "Pyranodipyran Derivatives with Tyrosyl DNA Phosphodiesterase 1 Inhibitory Activities and Fluorescent Properties from Aspergillus sp. EGF 15-0-3" Marine Drugs 20, no. 3: 211. https://doi.org/10.3390/md20030211