Developing a Chromochloris zofingiensis Mutant for Enhanced Production of Lutein under CO2 Aeration
Abstract
:1. Introduction
2. Results
2.1. Isolation of a “Stay-Green” Mutant of C. zofingiensis
2.2. A Nonsense Mutation Occurred in PKG Gene of Cz-pkg
2.3. Glucose Differentially Regulates the Biosynthesis of Photosynthetic Pigments
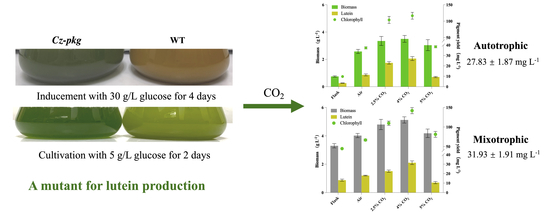
2.4. The Growth of Cz-pkg under Different Trophic Modes
2.5. Supplemented CO2 Promotes Cell Growth and Lutein Production
3. Discussion
4. Materials and Methods
4.1. Microalgae Strain and Cultivation
4.2. Mutant Generation, Selection, and Identification
4.3. Pigment Extraction and Analysis
4.4. DNA Extraction and Molecular Characterization of Mutant
4.5. Transcriptome Sequencing and Analysis of Differentially Expressed Genes
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Chen, F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.-S.; Cho, K.; Cho, D.-H.; Lee, Y.J.; Kim, H.-S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Přibyl, P.; Pilný, J.; Cepák, V.; Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Investigation of Chlorella vulgaris UTEX 265 Cultivation under Light and Low Temperature Stressed Conditions for Lutein Production in Flasks and the Coiled Tree Photo-Bioreactor (CTPBR). Appl. Biochem. Biotechnol. 2017, 183, 652–671. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, Y.; Wang, X.; Liu, J. Novel insights into salinity-induced lipogenesis and carotenogenesis in the oleaginous astaxanthin-producing alga Chromochloris zofingiensis: A multi-omics study. Biotechnol. Biofuels 2020, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Roth, M.S.; Westcott, D.J.; Iwai, M.; Niyogi, K.K. Hexokinase is necessary for glucose-mediated photosynthesis repression and lipid accumulation in a green alga. Commun. Biol. 2019, 2, 347. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and beta-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Gallaher, S.D.; Westcott, D.J.; Iwai, M.; Louie, K.B.; Mueller, M.; Walter, A.; Foflonker, F.; Bowen, B.P.; Ataii, N.N.; et al. Regulation of Oxygenic Photosynthesis during Trophic Transitions in the Green Alga Chromochloris zofingiensis. Plant Cell 2019, 31, 579–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Zhan, X.; Yang, P.; Li, J.; Chen, J.; Tang, B.; Wang, X.; Hong, Y. Dual Activities of Plant cGMP-Dependent Protein Kinase and Its Roles in Gibberellin Signaling and Salt Stress. Plant Cell 2019, 31, 3073–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassot, A.; Chauvin, M.A.; Bendridi, N.; Ji-Cao, J.; Vial, G.; Monnier, L.; Bartosch, B.; Alves, A.; Cottet-Rousselle, C.; Gouriou, Y.; et al. Regulation of Mitochondria-Associated Membranes (MAMs) by NO/sGC/PKG Participates in the Control of Hepatic Insulin Response. Cells 2019, 8, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldschmidt-Clermont, M.; Bassi, R. Sharing light between two photosystems: Mechanism of state transitions. Curr. Opin. Plant Biol. 2015, 25, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Reinbothe, C.; El Bakkouri, M.; Buhr, F.; Muraki, N.; Nomata, J.; Kurisu, G.; Fujita, Y.; Reinbothe, S. Chlorophyll biosynthesis: Spotlight on protochlorophyllide reduction. Trends Plant Sci. 2010, 15, 614–624. [Google Scholar] [CrossRef]
- Pruzinska, A.; Tanner, G.; Anders, I.; Roca, M.; Hortensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Liao, Q.; Xia, A.; Fu, Q.; Zhu, X. Photo-bioreactor design for microalgae: A review from the aspect of CO2 transfer and conversion. Bioresour. Technol. 2019, 292, 121947. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dash, S.K.; Sen, R. Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: A biorefinery model for healthcare, energy and environment. RSC Adv. 2015, 5, 73381–73394. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244 Pt 1, 664–671. [Google Scholar] [CrossRef]
- Yeh, T.-J.; Tseng, Y.-F.; Chen, Y.-C.; Hsiao, Y.; Lee, P.-C.; Chen, T.-J.; Chen, C.-Y.; Kao, C.-Y.; Chang, J.-S.; Chen, J.-C.; et al. Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res. 2017, 21, 103–119. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Li, Y.-T.; Huang, J.-C.; Chen, F. Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process. Biochem. 2008, 43, 1288–1292. [Google Scholar] [CrossRef]
- Mario, O.B.; Luis, M.C.; Ming, H.L.; Juan, M.D.; Gustavo, C.H. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Marondedze, C.; Groen, A.J.; Thomas, L.; Lilley, K.S.; Gehring, C. A Quantitative Phosphoproteome Analysis of cGMP-Dependent Cellular Responses in Arabidopsis thaliana. Mol. Plant 2016, 9, 621–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, W.; Wang, X.; Yang, L.; Hu, Y.; Wei, Y.; Liang, X.; Mao, L.; Bi, Y. Cyclic GMP is involved in auxin signalling during Arabidopsis root growth and development. J. Exp. Bot. 2014, 65, 1571–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Sun, H.; Deng, J.; Zhang, Y.; Li, Y.; Huang, J.; Chen, F. Coordinating Carbon Metabolism and Cell Cycle of Chlamydomonasreinhardtii with Light Strategies under Nitrogen Recovery. Microorganisms 2021, 9, 2480. [Google Scholar] [CrossRef]
- Ye, Y.; Huang, J.C. Defining the biosynthesis of ketocarotenoids in Chromochloris zofingiensis. Plant Divers. 2020, 42, 61–66. [Google Scholar] [CrossRef]
- Kang, M.; Yang, J.S.; Kim, Y.; Kim, K.; Choi, H.; Lee, S.H. Comparison of DNA extraction methods for drug susceptibility testing by allele-specific primer extension on a microsphere-based platform: Chelex-100 (in-house and commercialized) and MagPurix TB DNA Extraction Kit. J. Microbiol. Methods 2018, 152, 105–108. [Google Scholar] [CrossRef]
- Roth, M.S.; Cokus, S.J.; Gallaher, S.D.; Walter, A.; Lopez, D.; Erickson, E.; Endelman, B.; Westcott, D.; Larabell, C.A.; Merchant, S.S.; et al. Chromosome-level genome assembly and transcriptome of the green alga Chromochloris zofingiensis illuminates astaxanthin production. Proc. Natl. Acad. Sci. USA 2017, 114, E4269–E4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]






| Cultivation Conditions | Pigment Composition (mg g−1 DW) | |||
|---|---|---|---|---|
| Total Carotenoids | Lutein | Chl a | Chl b | |
| Autotrophy | ||||
| flasks | 5.32 ± 0.31 | 4.63 ± 0.22 | 10.63 ± 0.97 | 2.43 ± 0.30 |
| Bubble tubes + Air | 5.28 ± 0.22 | 4.40 ± 0.37 | 11.06 ± 1.01 | 3.41 ± 0.11 |
| Bubble tubes + 2.5% CO2 | 7.12 ± 0.80 | 5.70 ± 0.20 | 23.44 ± 2.05 | 7.56 ± 1.08 |
| Bubble tubes + 4.0% CO2 | 8.40 ± 0.81 | 7.73 ± 0.52 | 25.50 ± 2.44 | 6.86 ± 0.41 |
| Bubble tubes + 5.0% CO2 | 3.78 ± 0.61 | 3.06 ± 0.19 | 10.38 ± 1.20 | 2.25 ± 0.06 |
| Mixotrophy | ||||
| flasks | 5.94 ± 0.33 | 3.97 ± 0.33 | 11.37 ± 0.24 | 2.18 ± 0.50 |
| Bubble tubes + Air | 5.83 ± 0.34 | 4.47 ± 0.10 | 12.94 ± 0.41 | 3.67 ± 0.23 |
| Bubble tubes + 2.5% CO2 | 6.72 ± 0.35 | 4.71 ± 0.25 | 17.83 ± 1.00 | 5.02 ± 0.14 |
| Bubble tubes + 4.0% CO2 | 7.82 ± 0.49 | 6.28 ± 0.57 | 20.80 ± 1.32 | 6.93 ± 0.20 |
| Bubble tubes + 5.0% CO2 | 5.67 ± 0.25 | 2.52 ± 0.28 | 15.97 ± 1.30 | 3.46 ± 0.51 |
| Conditions | Lutein Contents (mg g−1 DW) | Pmax (mg L−1 day−1) | |||
|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | Day 8 | ||
| Auto + 2.5% CO2 | 2.16 ± 0.06 | 2.68 ± 0.14 | 3.25 ± 0.33 | 5.70 ± 0.35 | 5.45 ± 0.52 |
| Auto + 4.0% CO2 | 2.50 ± 0.25 | 2.77 ± 0.08 | 3.81 ± 0.40 | 7.73 ± 0.52 | 8.80 ± 0.60 |
| Mixo + 2.5% CO2 | 2.02 ± 0.13 | 2.40 ± 0.22 | 2.98 ± 0.21 | 4.71 ± 0.25 | 5.61 ± 0.44 |
| Mixo + 4.0% CO2 | 1.98 ± 0.16 | 2.85 ± 0.16 | 3.45 ± 0.17 | 6.28 ± 0.57 | 10.57 ± 0.73 |
| Sources | Lutein Content | Productivity (mg L−1 day−1) | References |
|---|---|---|---|
| Tagetes erecta (Marigold flower) | 0.17–5.70 mg g−1 | - | [3] |
| Chicken egg yolk | 16.22 μg g−1 | - | [25] |
| Brassica oleracea (Broccoli) | 39 μg g−1 | - | [25] |
| Tetracystis intermedium | 3.5 mg g−1 | - | [25] |
| Chlorella sorokiniana FZU60 | 11.22 mg g−1 | 8.25 | [1] |
| Chlorella vulgaris CS-41 | 9.0 mg g−1 | 1.56 | [1] |
| Chlorella sp. GY-H4 | 8.9 mg g−1 | 10.50 | [1] |
| Chlorella sorokiniana MB-1-M12 | 7.39 mg g−1 | 3.43 | [19] |
| Chlorella minutissima MCC-27 | 7.05 mg g−1 | 6.34 | [19] |
| Chlorella vulgaris | 5–9 mg g−1 | 1.60 | [19] |
| Chlorella sorokiniana AK-1 | 4.56 mg g−1 | 3.56 | [19] |
| Chromochloris zofingiensis WT | 3.07 mg g−1 | 1.24 | This study |
| Cz-pkg | 7.73 mg g−1 | 10.57 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Deng, J.; Lin, Y.; Huang, J.; Chen, F. Developing a Chromochloris zofingiensis Mutant for Enhanced Production of Lutein under CO2 Aeration. Mar. Drugs 2022, 20, 194. https://doi.org/10.3390/md20030194
Ren Y, Deng J, Lin Y, Huang J, Chen F. Developing a Chromochloris zofingiensis Mutant for Enhanced Production of Lutein under CO2 Aeration. Marine Drugs. 2022; 20(3):194. https://doi.org/10.3390/md20030194
Chicago/Turabian StyleRen, Yuanyuan, Jinquan Deng, Yan Lin, Junchao Huang, and Feng Chen. 2022. "Developing a Chromochloris zofingiensis Mutant for Enhanced Production of Lutein under CO2 Aeration" Marine Drugs 20, no. 3: 194. https://doi.org/10.3390/md20030194