A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
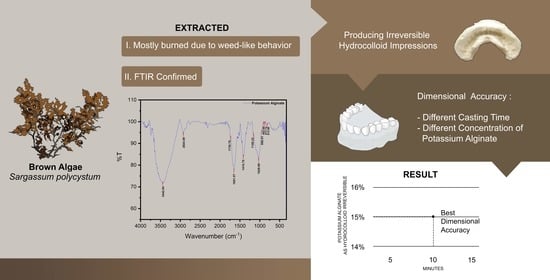
4.1. Extraction of Potassium Alginate from Sargassum polycystum
4.2. Purity Test by FTIR Analysis
4.3. Producing Irreversible Hydrocolloid Impression Material
4.4. Impression Procedure
4.5. Casting Procedure
4.6. Model Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imbery, T.A.; Nehring, J.; Janus, C.; Moon, P.C. Accuracy and dimensional stability of extended-pour and conventional alginate impression materials. J. Am. Dent. Assoc. 2010, 141, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Sharif, R.A.; Abdelaziz, K.M.; Alshahrani, N.M.; Almutairi, F.S.; Alaseri, M.A.; Abouzeid, H.L.; Elagib, M.F.A. The accuracy of gypsum casts obtained from the disinfected extended-pour alginate impressions through prolonged storage times. BMC Oral Health 2021, 21, 296. [Google Scholar] [CrossRef]
- Mudliar, V.L.; Li, K.C.; van Vuuren, L.J.; Waddell, J.N. The influence of undercut depths on the accuracy of casts poured from irreversible hydrocolloid impression materials. Heliyon 2020, 6, e03143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manar, J. Alginate as impression material. IJADS 2018, 4, 300–303. [Google Scholar]
- Damodara, E.K.; Litaker, M.S.; Rahemtulla, F.; McCracken, M.S. A randomized clinical trial to compare diagnostic casts made using plastic and metal trays. J. Prosthet. Dent. 2010, 104, 364–371. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs 2018, 17, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliu, M.P.; Sachelarie, L.; Romila, L.E.; Shardi, A.; Stadoleanu, C.; Tomita, D.I. Rheological Properties of Some Materials Used for Dental Impression. J. Biomim. Biomater. Biomed. Eng. 2017, 34, 75–81. [Google Scholar] [CrossRef]
- Madhavan, S. A review on hydrocolloids-agar and alginate. J. Pharm. Sci. Res. 2015, 7, 704. [Google Scholar]
- Garrofé, A.B.; Ferrari, B.A.; Picca, M.; Kaplan, A.E. Linear Dimensional Stability of Irreversible Hydrocolloid Materials Over Time. Acta Odontol. Latinoam. 2015, 28, 258–262. [Google Scholar]
- Nassar, U.; Aziz, T.; Flores-Mir, C. Dimensional stability of irreversible hydrocolloid impression materials as a function of pouring time: A systematic review. J. Prosthet. Dent. 2011, 106, 126–133. [Google Scholar] [CrossRef]
- Rohanian, A.; Ommati Shabestari, G.; Zeighami, S.; Samadi, M.J.; Shamshiri, A.R. Effect of storage time of extended-pour and conventional alginate impressions on dimensional accuracy of casts. J. Dent. 2014, 11, 655–664. [Google Scholar]
- Patel, R.D.; Kattadiyil, M.T.; Goodacre, C.J.; Winer, M.S. An in vitro investigation into the physical properties of irreversible hydrocolloid alternatives. J. Prosthet. Dent. 2010, 104, 325–332. [Google Scholar] [CrossRef]
- Ikram, F.; Michael, J.; Omer, R. Accuracy of some Elastic Impression Materials Used in Prosthetic Dentistry. Sulaimani Dent. J. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Effect of Powder/Water Ratio Variation on Viscosity, Tear Strength and Detail Reproduction of Dental Alginate Impression Material (In Vitro and Clinical Study). Polymers 2021, 13, 2923. [Google Scholar] [CrossRef] [PubMed]
- Hamrun, N.; Taufiq, A.; Tahir, D. Studies on Surface Morphology of Irreversible Hydrocolloid Impression Material Based on Brown Algae Type Padina sp. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kazimierz Dolny, Poland, 21–23 November 2019; p. 012025. [Google Scholar]
- Reyes-Tisnado, R.; Hernández-Carmona, G.; López-Gutiérrez, F.; Vernon-Carter, E.J.; Castro-Moroyoqui, P. Sodium and potassium alginates extracted from Macrocystis pyrifera algae for use in dental impression materials. Cienc. Mar. 2004, 30, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Chee, S.-Y.; Wong, P.-K.; Wong, C.-L. Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Kim, S.J.; Lee, Y.M. pH/temperature-responsive semi-IPN hydrogels composed of alginate and poly (N-isopropylacrylamide). J. Appl. Polym. Sci. 2002, 83, 1128–1139. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J. Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [Green Version]
- McCabe, J.F.; Walls, A.W. Applied Dental Materials; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Guiraldo, R.D.; Berger, S.B.; Consani, R.L.; Consani, S.; de Carvalho, R.V.; Lopes, M.B.; Meneghel, L.L.; da Silva, F.B.; Sinhoreti, M.A. Characterization of morphology and composition of inorganic fillers in dental alginates. BioMed Res. Int. 2014, 2014, 178064. [Google Scholar] [CrossRef]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bono, A.; Anisuzzaman, S.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Taira, M.; Araki, Y. DTG thermal analyses and viscosity measurements of three commercial agar impression materials. J. Oral Rehabil. 2002, 29, 697–701. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wassell, R.W.; Barker, D.; Walls, A.W. Crowns and other extra-coronal restorations: Impression materials and technique. Br. Dent. J. 2002, 192, 679–684, 687–690. [Google Scholar] [CrossRef]
- Manappallil, J.J. Basic Dental Materials; JP Medical Ltd.: Mangalore, India, 2015. [Google Scholar]
- Skinner, E.; Cooper, E.; Beck, F.E. Reversible and irreversible hydrocolloid impression materials. J. Am. Dent. Assoc. 1950, 40, 196–207. [Google Scholar] [CrossRef]
- Mousavi, S.; Rahbar, M.; Rostamzadeh, F.; Jafaria, K.; Hekmatfar, S. Dimensional stability of casts derived from three types of alginate at different times after impression. Pesqui. Bras. Odontopediatria Clínica Integr. 2019, 19, e4137. [Google Scholar] [CrossRef]
- Kurtulus, K.; Tüfekci, K. Empirical study of alginate impression materials by customized proportioning system. J. Adv. Prosthodont. 2016, 8, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, G.; Murata, H.; Li, Y.-A.; Hamada, T. Physical properties and additional characteristics of current elastomeric impression materials. Int. Chin. J. Dent. 2005, 5, 80–90. [Google Scholar]
- Hamalian, T.A.; Nasr, E.; Chidiac, J.J. Impression materials in fixed prosthodontics: Influence of choice on clinical procedure. J. Prosthodont. 2011, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]


| Peak Wavelengths for Potassium Alginate Extracted from Sargassum polycystum (cm−1) | Functional Group Interpretation | Wavelength Reference (cm−1) |
|---|---|---|
| 3442.94 | Hydroxyl (-OH) | 3700–3100 |
| 2924.09 | C-H aliphatic | 3000–2800 |
| 1739.79 | Carbonyl (C=O) | 1870–1650 |
| 1651.07 | COO-asymmetric | 1600–1590 |
| 1415.75 | COO-symmetrical | 1410 |
| 1029.99 | C-OH | 1300–1000 |
| 1159.22 | C-O-C | 1250–1170 |
| 813.96; 873.75; 902.69 | Guluronic fingerprints | 890–900 |
| 950.91 | C-O stretching Uronic acid | 948 |
| Casting Time (Minutes) | Mesiodistal Width | Mesiodistal Width | Mesiodistal Width | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA 14% (mm) | Control (mm) | Mean Difference | PA 15% (mm) | Control (mm) | Mean Difference | PA 16% (mm) | Control (mm) | Mean Difference | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Immediately | 8.209 ± 0.267 | 7.996 ± 0.005 | 0.213 | 8.197 ± 0.001 | 7.996 ± 0.005 | 0.201 | 8.056 ± 0.561 | 7.996 ± 0.005 | 0.060 | 0.148 |
| 5 | 8.124 ± 0.133 | 7.988 ± 0.010 | 0.135 | 8.104 ± 0.119 | 7.988 ± 0.010 | 0.115 | 7.971 ± 0.053 | 7.988 ± 0.010 | −0.017 | 0.084 |
| 10 | 7.940 ± 0.254 | 7.985 ± 0.005 | −0.045 | 7.993 ± 0.441 | 7.985 ± 0.005 | 0.008 | 7.961 ± 0.149 | 7.985 ± 0.005 | −0.023 | 0.715 |
| 15 | 7.914 ± 0.147 | 7.978 ± 0.009 | −0.065 | 7.822 ± 0.183 | 7.978 ± 0.009 | −0.156 | 7.956 ± 0.067 | 7.978 ± 0.009 | −0.022 | 0.381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamrun, N.; Talib, B.; Ruslin, M.; Pangeran, H.; Hatta, M.; Marlina, E.; Yusuf, A.S.H.; Saito, T.; Ou, K.-L. A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material. Mar. Drugs 2022, 20, 55. https://doi.org/10.3390/md20010055
Hamrun N, Talib B, Ruslin M, Pangeran H, Hatta M, Marlina E, Yusuf ASH, Saito T, Ou K-L. A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material. Marine Drugs. 2022; 20(1):55. https://doi.org/10.3390/md20010055
Chicago/Turabian StyleHamrun, Nurlindah, Bahruddin Talib, Muhammad Ruslin, Hasminar Pangeran, Mochammad Hatta, Erni Marlina, Andi Sitti Hajrah Yusuf, Takashi Saito, and Keng-Liang Ou. 2022. "A Promising Potential of Brown Algae Sargassum polycystum as Irreversible Hydrocolloid Impression Material" Marine Drugs 20, no. 1: 55. https://doi.org/10.3390/md20010055