Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems
Abstract
:1. Chitosan
2. Plant-Derived Biomolecules
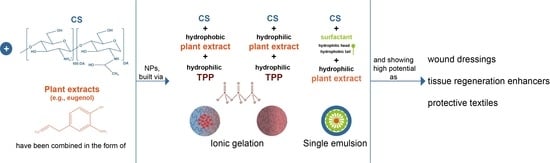
3. Chitosan-Based Small-Scaled Particles Loaded with Plant-Derived Biomolecules
4. Biomedical Applications: Fiber-Based Systems
- (a)
- (b)
- (c)
| Fiber-Based Structure | Immobilization Strategy | Loaded Carrier | Main Chemical Bonds between Carrier and Fiber | Bioactivity | Potential Application | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Materials | Processing | Functional Groups | Composition | Plant Extract | Preparation Method | |||||
| Collagen/fibrin | Cryodesiccation | - | Dispersion (solubilization until NP homogenization was reached within the polymeric solution) | CS/TPP | Gallic acid | Ionic gelation | - | At 3, 6, 24, and 72 h, 9.71 ± 2.3%, 20.69 ± 3.9%, ≈41% and ≈72% of gallic acid was released from the scaffolds. The engineered scaffold accelerated angiogenesis, hexosamine synthesis, collagen deposition and recruited immune cells at wound area. | Wound healing | [104] |
| PCL/CS/Curcumin | Electrospinning | - | Electrospraying PCL/CS/curcumin nanofibers with curcumin-loaded CS NPs | CS/TPP | Curcumin | Ionic gelation | - | Improved antibacterial, antioxidant, and cell proliferation efficiencies, with higher swelling capability and water vapor transition rate of the electrospun fibers. In vivo examination showed significant improvement of wound healing in MRSA-infected wounds. | Wound healing | [172] |
| PCL/GN | Electrospinning | - | Dispersion (solubilization until NP homogenization was reached within the polymeric solution) | CS/TPP | Curcumin | Ionic gelation | - | Improved biocompatibility and wound healing abilities in a full-thickness excisional animal model. Cell attachment and proliferation was enhanced in the presence of the NPs. | Wound healing and skin substitutes | [181] |
| PCL/PVP | Co-axial electrospinning (sheath PCL and core PVP) | - | Solubilization of the NPs with PVP portion of the fiber and extrusion as the core of the electrospun fibers | CS/TPP | Veratric acid | Ionic gelation | - | Reached 60% release after 20 days of incubation. Modified fibers were biocompatible with mouse mesenchymal stem cells, promoting their differentiation (upregulation of bone differentiation-related markers). | Bone regeneration | [183] |
| PCL | Electrospinning | - | Dispersion (solubilization until NP homogenization was reached within the polymeric solution) | CS/TPP | Sinapic acid | Ionic gelation | - | Enhanced osteoblast differentiation and activated the osteogenesis signaling pathways in mouse mesenchymal stem cells. In vivo data reflected the extract ability to instigate bone formation. | Bone regeneration | [184] |
| Wool | - | -OH | Pad-dry-cure technique | CS/TPP | Propolis | Ionic gelation | Hydrogen bonding and physisorption. | Enhanced antimicrobial action against fungi and bacteria. Synergistic effects with textile dyes (improved antimicrobial protection). | Textile finishes for microbial-protective clothing | [189] |
| Cotton | Dip in 3% NaOH for 45 min, soaked in 10 g·L−1 sodium dodecyl sulphate for 30 min and in hot ethanol for 30 min. Then, washed with boiling ultrapure water for 5 times and dried at 25 °C under 65% relative humidity for use. Prior to surface modification, fabric degassed by negative pressure | -OH | Immersion in particle dispersion at 40 °C, 100 rpm/min for 1 h. Wet pick up of 100%. The finished textile was dipped into deionized water and placed into constant temperature and pressure to dry the textile and remove the extra water. | CS, citric acid, CO-40, TGI or CS, citric acid, Span 80, Tween 80 | Citronella oil | Emulsification and ionic gelation | Hydrogen bonding between particles and textile fibers, and electrostatic interaction with -NH2 of CS | Aromatic retention of 28.84% after 10 washing cycles. | Aromatic textile finishing | [186] |
| Cotton | Non-ionic detergent used at 25 °C for 30 min for fabric washing, warm water then cold water applied, and finally, fabric drying | -OH | Dip-dry-cure: Immersion in 100 g/L of gel on a shaker at 1000 rpm at 25 °C for 2 h, dried at 50 °C for 5 min and cured at 100 °C for 2 min, rinsing with water to remove unbound or loosely bounded molecules. | CS, Tween 80, TPP/acrylate | Lemongrass oil | Emulsification followed by ionic gelation. Acrylate added as fabric adhesive | Hydrogen bonding between particles and textile fibers, and electrostatic interaction with -NH2 of CS | 100% of repellency against mosquitoes (75% after 15 washes). Absence of dermal toxicity in mice. | Insect-repellent clothing | [187] |
| Cotton | Perfumed cotton fabrics initially washed with water at 40 °C, drained and rinsed with water at 25 °C and finally spun. | -OH | Impregnation: immersion in particle dispersion for 2 h under vacuum (100 Pa) at 30 °C, air-drying at 50 °C with the air current rate of 0.4 m/s for 1 h in the oven (moisture content: 0.01103 kg/m3). | CS, Tween 80/TPP | Rose fragrance | Emulsification followed by ionic gelation | Hydrogen bonding between particles and textile fibers, and electrostatic interaction with -NH2 of CS | 80% plant extract release in 20 washing cycles. 55% release in 10 days at 70 °C, 0.4 m/s of air current rate and moisture content of 0.01 kg/m3. | Long-term fragrance-releasing textiles | [190] |
| Cotton | - | -OH | Dip-pad-dry-cure method, with fabric immersed in carrier dispersion and citric acid binder (1%) for 5 min, padded 15 m/min with a pressure of 1 kgf/cm2, air-dried, cured 3 min at 140 °C and immersed 5 min in sodium lauryl sulfate to remove unbound NPs and the soap solution, followed by air-drying. | CS, Tween 80, Span 80, palm oil and TPP | Neem methanolic extract | Emulsification followed by ionic gelation | Esterification with -COOH of citric acid also promoting electrostatic interaction with -NH2 of CS | Enhanced antibacterial efficiency (until 20 laundry washes): 100% S. aureus reduction (ZoI: 20 mm) and 93% E. coli reduction (ZoI: 14 mm). | Textile finishes for bacterial protective clothing | [180] |
| Cotton | - | -OH | Dip-pad-dry-cure: immersion in particle dispersion and citric acid binder for 5 min, padding mangle to remove excess solution, with 100% wet pick-up, air-drying, curing at 140 °C for 3 min, immersion in sodium lauryl sulfate for 5 min to remove unbound extract, rinsing to remove the soap solution and air-drying. | Alginate, CaCl2, CS | Methanol extracts of Ocimum sanctum | Ionic gelation and polyelectrolyte complexation | Esterification with -COOH of citric acid also promoting electrostatic interaction with -NH2 of CS | 100% (B. cereus, P. aeruginosa, and S. aureus) or 98% (E. coli) bacterial reduction, effective until 20 or 10 washing cycles. | Biocontrol agent against bacteria in fabrics | [191] |
| Cotton | Fabric washed 0, 5, 10, 15, and 25 times, washing with 2% soapy water for 15 min, and rinsing in clean water | -OH | Dip-dry-cure: immersion in bath containing microcapsule emulsion, 2D resin, catalytic agent, and JFC penetrant. Wet pick up at 100%, drying at 80 °C for 3 min, curing at 160 °C for 2 min, and then cooling down to room temperature. Washing and drying. | CS, gelatin, span-80, glutaraldehyde | Patchouli oil | Emulsification and chemical crosslinking | Crosslinking between 2D resin and hydroxyl groups of cotton and/or microcapsules through acid-catalyzed dehydration | Gradual decrease of antibacterial activity down to 75 and 70% (against S. aureus and E. coli, respectively) after 25 washes. | Antibacterial mask, bacteriostatic sheet and health-care clothes | [192] |
| Cotton | Textile binder (Knittex CHN, melamine resin) used to enhance microcapsule fixation to the fabric | -NH2 | Dip-pad-dry-cure: immersion in microcapsule solution, vertical padding 1.5 kg/cm2 and 7.5 rpm with two dips and two nips, drying at 80 °C for 3 min, curing in a Mathis curing oven at 100 °C for 3 min, and air-drying. | CS, alginate, liquid paraffin, Span 80, NaOH, glutaraldehyde | PentaHerbs aqueous extracts | Polyelectrolyte complexation, emulsification, and chemical crosslinking | Electrostatic interaction of -NH2 of melamine resin and -COOH of alginate | Cytocompatible towards human epidermal equivalent. | Garment development for atopic dermatitis | [193] |
| Cotton | - | -OH | Dip-pad-dry-cure: immersion in microcapsule dispersion, sodium hypophosphite (catalyst), citric acid, and deionized water (bath ratio = 1:20) for 70 min; rolling (two dips and two rollings; wet pick up, 80%; pressure, 0.3 MPa). Drying at 90 °C for 3 min, curing at 160 °C for 2 min, then cooling to room temperature. Washing with water and drying under vacuum at 60 °C for 24 h. | CS, citric acid | Vanillin ethanolic solution | Emulsification and ionic gelation | Esterification with -COOH of citric acid also promoting electrostatic interaction with -NH2 of CS | Sustained drug release until 14 laundry washes. | Functional fibers in the textile industry | [194] |
| Cellulose | Fibers washed with 1% non-ionic detergent at 30 °C for 30 min and rinsed with water for 15 min | -OH | Dip-pad-dry: immersion in particle dispersion, padding at 2.5 m/min and 4 bars to remove excess solution, air-drying, rinsing with deionized water, and air-drying again. | CS, surfactant, NaOH | Limonene oil | Emulsification and neutralization | Hydrogen bonding between particles and textile fibers, and electrostatic interaction with -NH2 of CS | Decreased oil volatility in 8 h. | Insect repellent for textiles | [195] |
| Cotton | - | -OH | Pad-dry: padding at 35 rpm for 5 min and drying at 60 °C for 10 min | CS | Aloe vera herbal nanopowder | Coating | Hydrogen bonding between particles and textile fibers, and electrostatic interaction with -NH2 of CS | ZoI of 22 mm and 27 mm against E. coli and S. aureus, respectively, UV-protection factor of 57 and superhydrophobicity 155°. | Antibacterial protective clothing | [188] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Antunes, J.; Gonçalves, R.; Barbosa, M. Chitosan/poly(γ-glutamic acid) polyelectrolyte complexes: From self-assembly to application in biomolecules delivery and regenerative medicine. Res. Rev. J. Mater. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Amaral, I.F.; Lamghari, M.; Sousa, S.R.; Sampaio, P.; Barbosa, M.A. Rat bone marrow stromal cell osteogenic differentiation and fibronectin adsorption on chitosan membranes: The effect of the degree of acetylation. J. Biomed. Mater. Res. Part A 2005, 75, 387–397. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun nanocomposites containing cellulose and its derivatives modified with specialized biomolecules for an enhanced wound healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, M. Physical Properties of Chitosan and Derivatives in Sol and Gel States. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 23–43. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.C.; De Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Halim, A.S.; Keong, L.C.; Zainol, I.; Rashid, A.H.A. Biocompatibility and Biodegradation of Chitosan and Derivatives. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 57–73. [Google Scholar] [CrossRef]
- Anitha, A.; Rejinold, S.N.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Approaches for Functional Modification or Cross-Linking of Chitosan. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 107–124. [Google Scholar] [CrossRef] [Green Version]
- Guan, G.; Abul Kalam Azad, M.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, C.; Sousa, F.; Sarmento, B. Chitosan-based nanomedicine for brain delivery: Where are we heading? React. Funct. Polym. 2020, 146. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Huang, B.Q.; Tsai, G.J.; Hsieh, H.Y.; Sung, H.W.; Mi, F.L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Amaral, I.F.; Águas, A.P.; Barbosa, M.A. Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffolds. J. Biomed. Mater. Res. A 2010, 93, 20–28. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; de Torre-Minguela, C.; Gomez, A.I.; Águas, A.P.; Barbosa, M.A.; Pelegrín, P.; Barbosa, J.N. 3D chitosan scaffolds impair NLRP3 inflammasome response in macrophages. Acta Biomater. 2019, 91, 123–134. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Águas, A.P.; Barbosa, J.N. Macrophage polarization following chitosan implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Gonçalves, R.M.; Antunes, J.C.; Pinto, M.L.; Pinto, A.T.; Castro, F.; Monteiro, C.; Barbosa, M.A.; Oliveira, M.J. An interferon-γ-delivery system based on chitosan/poly(γ-glutamic acid) polyelectrolyte complexes modulates macrophage-derived stimulation of cancer cell invasion in vitro. Acta Biomater. 2015, 23, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Pinto, M.L.; Almeida, R.; Pereira, F.; Silva, A.M.; Pereira, C.L.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Chitosan/poly(γ-glutamic acid) nanoparticles incorporating IFN-γ for immune response modulation in the context of colorectal cancer. Biomater. Sci. 2019, 7, 3386–3403. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Pinto, M.L.; Silva, A.M.; Pereira, C.L.; Teixeira, G.Q.; Gomez-Lazaro, M.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Pro-inflammatory chitosan/poly(γ-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomater. 2017, 63, 96–109. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Selma-Royo, M.; Simal-Gandara, J.; Collado, M.C.; Barba, F.J. Potential benefits of high-added-value compounds from aquaculture and fish side streams on human gut microbiota. Trends Food Sci. Technol. 2021, 112, 484–494. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, H.; Yang, C.; Du, H.; Wang, H.; Tu, J. Effects of chitosan nanoparticle supplementation on growth performance, humoral immunity, gut microbiota and immune responses after lipopolysaccharide challenge in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 597–605. [Google Scholar] [CrossRef]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of dysbiotic human gut microbiome for homeostasis. Life Sci. 2021, 278. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138. [Google Scholar] [CrossRef]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kaolaor, A.; Phunpee, S.; Ruktanonchai, U.R.; Suwantong, O. Effects of β-cyclodextrin complexation of curcumin and quaternization of chitosan on the properties of the blend films for use as wound dressings. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Dornish, M.; Kaplan, D.S.; Arepalli, S.R. Regulatory Status of Chitosan and Derivatives. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 463–481. [Google Scholar] [CrossRef]
- Deng, P.; Chen, J.; Yao, L.; Zhang, P.; Zhou, J. Thymine-modified chitosan with broad-spectrum antimicrobial activities for wound healing. Carbohydr. Polym. 2021, 257. [Google Scholar] [CrossRef]
- Davani, F.; Alishahi, M.; Sabzi, M.; Khorram, M.; Arastehfar, A.; Zomorodian, K. Dual drug delivery of vancomycin and imipenem/cilastatin by coaxial nanofibers for treatment of diabetic foot ulcer infections. Mater. Sci. Eng. C 2021, 123. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohydr. Polym. 2021, 256. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-containing essential oils loaded onto chitosan/polyvinyl alcohol blended films and their ability to eradicate Staphylococcus aureus or Pseudomonas aeruginosa from infected microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Sustainable development of mosquito-repellent, flame-retardant, antibacterial, fragrant and antioxidant linen using microcapsules containing Thymus vulgaris oil in in-situ generated chitosan-phosphate. Cellulose 2021, 28, 2599–2614. [Google Scholar] [CrossRef]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Ghosh, S.; Mukherjee, R.; Haldar, J. Quaternary Lipophilic Chitosan and Gelatin Cross-Linked Antibacterial Hydrogel Effectively Kills Multidrug-Resistant Bacteria with Minimal Toxicity toward Mammalian Cells. Biomacromolecules 2021, 22, 557–571. [Google Scholar] [CrossRef]
- Alborzi, Z.; Izadi-Vasafi, H.; Ghayoumi, F. Wound dressings based on chitosan and gelatin containing starch, sesame oil and banana peel powder for the treatment of skin burn wounds. J. Polym. Res. 2021, 28. [Google Scholar] [CrossRef]
- Prabha, S.; Sowndarya, J.; Ram, P.J.V.S.; Rubini, D.; Hari, B.N.V.; Aruni, W.; Nithyanand, P. Chitosan-Coated Surgical Sutures Prevent Adherence and Biofilms of Mixed Microbial Communities. Curr. Microbiol. 2021, 78, 502–512. [Google Scholar] [CrossRef]
- Yu, R.; Cornette de Saint-Cyr, L.; Soussan, L.; Barboiu, M.; Li, S. Anti-bacterial dynamic hydrogels prepared from O-carboxymethyl chitosan by dual imine bond crosslinking for biomedical applications. Int. J. Biol. Macromol. 2021, 167, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Yoon, S.J.; Koo, J.H.; Yoon, Y.; Byun, H.J.; Kim, H.S.; Khang, G.; Chun, H.J.; Yang, D.H. β-Cyclodextrin/Triclosan Complex-Grafted Methacrylated Glycol Chitosan Hydorgel by Photocrosslinking via Visible Light Irradiation for a Tissue Bio-Adhesive. Int. J. Mol. Sci. 2021, 22, 700. [Google Scholar] [CrossRef]
- Kodama, J.; Chen, H.; Zhou, T.; Kushioka, J.; Okada, R.; Tsukazaki, H.; Tateiwa, D.; Nakagawa, S.; Ukon, Y.; Bal, Z.; et al. Antibacterial efficacy of quaternized chitosan coating on 3D printed titanium cage in rat intervertebral disc space. Spine J. 2021. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Multifunctional Linen Fabric Obtained through Finishing with Chitosan-gelatin Microcapsules Loaded with Cinnamon Oil. J. Nat. Fibers 2021. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Li, S.; Kang, Q.; Zhang, R.; Zhu, Z. Self-assembled nanogels of luminescent thiolated silver nanoclusters and chitosan as bactericidal agent and bacterial sensor. Mater. Sci. Eng. C 2021, 118. [Google Scholar] [CrossRef]
- Tawfik, T.M.; El-Masry, A.M.A. Preparation of chitosan nanoparticles and its utilization as novel powerful enhancer for both dyeing properties and antimicrobial activity of cotton fabrics. Biointerface Res. Appl. Chem. 2021, 11, 13652–13666. [Google Scholar] [CrossRef]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C 2021, 120, 111786. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Bahador, A.; Partoazar, A. Combinatorial therapy of chitosan hydrogel-based zinc oxide nanocomposite attenuates the virulence of Streptococcus mutans. BMC Microbiol. 2021, 21. [Google Scholar] [CrossRef]
- Qiu, Y.; Dong, Y.; Zhao, S.; Zhang, J.; Huang, P.; Wang, W.; Dong, A.; Deng, L. N-dodecylated chitosan/graphene oxide composite cryogel for hemostasis and antibacterial treatment. J. Appl. Polym. Sci. 2021, 138, 50572. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ooi, C.W.; Song, C.P.; Wang, C.Y.; Liu, B.L.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Anand, M.; Quero, F. Highly efficient antibacterial activity of graphene/chitosan/magnetite nanocomposites against ESBL-producing Pseudomonas aeruginosa and Klebsiella pneumoniae. Colloids Surf. B 2021, 202, 111690. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical synthesis of chitosan/silver nanoparticles multilayer hydrogel coating with pH-dependent controlled release capability and antibacterial property. Colloids Surf. B 2021, 202, 111711. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Zhou, C.; Ao, H.Y.; Han, X.; Jiang, W.W.; Yang, Z.F.; Ma, L.; Deng, X.Y.; Wan, Y.Z. Engineering a novel antibacterial agent with multifunction: Protocatechuic acid-grafted-quaternized chitosan. Carbohydr. Polym. 2021, 258, 117683. [Google Scholar] [CrossRef]
- Da Silva, N.P.; Carmo Rapozo Lavinas Pereira, E.D.; Duarte, L.M.; de Oliveira Freitas, J.C.; de Almeida, C.G.; da Silva, T.P.; de Melo, R.C.N.; Morais Apolônio, A.C.; de Oliveira, M.A.L.; de Mello Brandão, H.; et al. Improved anti-Cutibacterium acnes activity of tea tree oil-loaded chitosan-poly(ε-caprolactone) core-shell nanocapsules. Colloids Surf. B 2020, 196, 111371. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Lv, J.; Wang, C.; Liu, Y. Preparation of thiolated chitosan/silver nanowire composite hydrogels with antimicrobial activity for obstetric wound care. Mater. Lett. 2020, 280, 128497. [Google Scholar] [CrossRef]
- Cele, Z.E.D.; Somboro, A.M.; Amoako, D.G.; Ndlandla, L.F.; Balogun, M.O. Fluorinated quaternary chitosan derivatives: Synthesis, characterization, antibacterial activity, and killing kinetics. ACS Omega 2020, 5, 29657–29666. [Google Scholar] [CrossRef]
- Ejaz, S.; Ihsan, A.; Noor, T.; Shabbir, S.; Imran, M. Mannose functionalized chitosan nanosystems for enhanced antimicrobial activity against multidrug resistant pathogens. Polym. Test. 2020, 91, 106814. [Google Scholar] [CrossRef]
- Chylińska, M.; Kaczmarek, H. N-Halamine Hydantoin-Containing Chitosan: Synthesis, Characterization, Thermal and Photolytic Stability Studies. Molecules 2020, 25, 3728. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Alihosseini, F.; Hosseini, S.A. Improving physical and biological properties of nylon monofilament as suture by Chitosan/Hyaluronic acid. Int. J. Biol. Macromol. 2020, 164, 3394–3402. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Fajardo, A.R.; Gerola, A.P.; Rodrigues, J.H.S.; Nakamura, C.V.; Muniz, E.C.; Hsieh, Y.L. First report of electrospun cellulose acetate nanofibers mats with chitin and chitosan nanowhiskers: Fabrication, characterization, and antibacterial activity. Carbohydr. Polym. 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.B.; Sarrafzadeh, M.H.; Salami, M.; Khorramizadeh, M.R. A pH-sensitive delivery system based on N-succinyl chitosan-ZnO nanoparticles for improving antibacterial and anticancer activities of curcumin. Int. J. Biol. Macromol. 2020, 151, 428–440. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-responsive chitosan hydrogel for healing of full-thickness wounds infected with XDR bacteria isolated from burn patients: In vitro and in vivo animal model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of natural fiber-reinforced biocomposites for biomedical applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Alsheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Alviano, D.S.; Alviano, C.S. Plant extracts: Search for new alternatives to treat microbial diseases. Curr. Pharm. Biotechnol. 2009, 10, 106–121. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Minatel, I.O.; Borges, C.; Ferreira, M.I.; Gomez Gomez, H.; Chen, O.; Lima, G. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar] [CrossRef] [Green Version]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.M.P.S.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential oils encapsulated in chitosan microparticles against Candida albicans biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan-alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, S.; Khoee, S.; Ghandi, M. Preparation and characterization of rod-like chitosan–quinoline nanoparticles as pH-responsive nanocarriers for quercetin delivery. Int. J. Biol. Macromol. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Khardrawy, Y.A.; Abd-El Daim, T.M.; Elfeky, A.S.; Abd Rabo, A.A.; Mustafa, A.B.; Mostafa, I.T. Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol. Behav. 2020, 222. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The antimicrobial agents. Sys. Rev. Pharm. 2016, 8, 62–70. [Google Scholar] [CrossRef]
- Eustáquio, A.S.; Gust, B.; Luft, T.; Li, S.M.; Chater, K.F.; Heide, L. Clorobiocin biosynthesis in Streptomyces: Identification of the halogenase and generation of structural analogs. Chem. Biol. 2003, 10, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Phutdhawong, W.; Chuenchid, A.; Taechowisan, T.; Sirirak, J.; Phutdhawong, W.S. Synthesis and Biological Activity Evaluation of Coumarin-3-Carboxamide Derivatives. Molecules 2021, 26, 1653. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Kumar Sahu, P.; Dehury, B.; Nath Padhy, R.; Kumar Paidesetty, S. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Erin Lim, S.H.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavada, B.S.; Osterne, V.J.S.; Pinto-Junior, V.R.; Nascimento, K.S. ConBr, the lectin from Canavalia brasiliensis mart. seeds: Forty years of research. Curr. Protein Pept. Sci. 2019, 20, 600–613. [Google Scholar] [CrossRef]
- Rüdiger, H.; Gabius, H.J. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconjug. J. 2002, 18, 589–613. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terras, F.R.G.; Schoofs, H.M.E.; Thevissen, K.; Osborn, R.W.; Vanderleyden, J.; Cammue, B.P.A.; Broekaert, W.F. Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2S albumins and by barley trypsin inhibitors. Plant Physiol. 1993, 103, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.A. Combination of drugs: An effective approach for enhancing the efficacy of antibiotics to combat drug resistance. In Antibacterial Drug Discovery to Combat MDR: Natural Compounds, Nanotechnology and Novel Synthetic Sources; Ahmad, I., Ahmad, S., Rumbaugh, K.P., Eds.; Springer: Singapore, 2019; pp. 427–440. [Google Scholar] [CrossRef]
- Kaparekar, P.S.; Pathmanapan, S.; Anandasadagopan, S.K. Polymeric scaffold of Gallic acid loaded chitosan nanoparticles infused with collagen-fibrin for wound dressing application. Int. J. Biol. Macromol. 2020, 165, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez y Gómez, Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: Characterization and antibacterial activity. Carbohydr. Polym. 2019, 226. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2005. [Google Scholar]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hamilton-Miller, J.M.T.; Hara, Y.; Nagaoka, Y.; Kumagai, A.; Uesato, S.; Taylor, P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, B.; Yazdiniapour, Z.; Sadeghi, M.; Akbari, M.; Troiano, R.; Lanzotti, V. Cinnamic acid derivatives from welsh onion (Allium fistulosum) and their antibacterial and cytotoxic activities. Phytochem. Anal. 2021, 32, 84–90. [Google Scholar] [CrossRef]
- Brownlee, H.E.; McEuen, A.R.; Hedger, J.; Scott, I.M. Anti-fungal effects of cocoa tannin on the witches’ broom pathogen Crinipellis perniciosa. Physiol. Mol. Plant Pathol. 1990, 36, 39–48. [Google Scholar] [CrossRef]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Al Shahrani, M.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196–204. [Google Scholar] [CrossRef]
- Gomes, C.L.; de Albuquerque Wanderley Sales, V.; Gomes de Melo, C.; Ferreira da Silva, R.M.; Vicente Nishimura, R.H.; Rolim, L.A.; Rolim Neto, P.J. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186. [Google Scholar] [CrossRef]
- Linzner, N.; Fritsch, V.N.; Busche, T.; Tung, Q.N.; Loi, V.V.; Bernhardt, J.; Kalinowski, J.; Antelmann, H. The plant-derived naphthoquinone lapachol causes an oxidative stress response in Staphylococcus aureus. Free Radic. Biol. Med. 2020, 158, 126–136. [Google Scholar] [CrossRef]
- Mone, N.S.; Bhagwat, S.A.; Sharma, D.; Chaskar, M.; Patil, R.H.; Zamboni, P.; Nawani, N.N.; Satpute, S.K. Naphthoquinones and their derivatives: Emerging trends in combating microbial pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Novais, J.S.; Carvalho, M.F.; Ramundo, M.S.; Beltrame, C.O.; Geraldo, R.B.; Jordão, A.K.; Ferreira, V.F.; Castro, H.C.; Figueiredo, A.M.S. Antibiofilm effects of N,O-acetals derived from 2-amino-1,4-naphthoquinone are associated with downregulation of important global virulence regulators in methicillin-resistant Staphylococcus aureus. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Tamokou, J.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Adnan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, M.K.; Anwar, H.; Sarfraz, I.; Riaz, A.; Manzoor, M.; Adem, Ş.; et al. Ginkgetin: A natural biflavone with versatile pharmacological activities. Food Chem. Toxicol. 2020, 145. [Google Scholar] [CrossRef]
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Enzymology and Molecular Biology. In Advances in Enzymology and Related Areas of Molecular Biology; John Wiley and Sons: Hoboken, NJ, USA, 1983; pp. 1–136. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.; Qi, M.; Sun, J.; Ai, M.; Ma, X.; Cai, W.; Zhou, Y.; Ni, L.; Hu, J.; Xu, F.; et al. Preparation, characterization and wound healing effect of vaccarin-chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 165, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Hadinoto, K. A potential quorum-sensing inhibitor for bronchiectasis therapy: Quercetin-chitosan nanoparticle complex exhibiting superior inhibition of biofilm formation and swimming motility of Pseudomonas aeruginosa to the native quercetin. Int. J. Mol. Sci. 2021, 22, 1541. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. BBA Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and flavan-3-ols in the prevention and treatment of periodontitis—Antibacterial effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Mahmoud, A.A.; Williams, H.J.; Scott, A.I.; Reibenspies, J.H.; Mabry, T.J. New sesquiterpene α-methylene lactones from the egyptian plant jasonia candicans. J. Nat. Prod. 1993, 56, 1276–1280. [Google Scholar] [CrossRef]
- Barre, J.T.; Bowden, B.F.; Coll, J.C.; De Jesus, J.; De La Fuente, V.E.; Janairo, G.C.; Ragasa, C.Y. A bioactive triterpene from Lantana camara. Phytochemistry 1997, 45, 321–324. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Waterman, P.G. A new antibacterial sesquiterpene from Premna Oligotricha. J. Nat. Prod. 1993, 56, 140–143. [Google Scholar] [CrossRef]
- Himejima, M.; Hobson, K.R.; Otsuka, T.; Wood, D.L.; Kubo, I. Antimicrobial terpenes from oleoresin of ponderosa pine tree Pinus ponderosa: A defense mechanism against microbial invasion. J. Chem. Ecol. 1992, 18, 1809–1818. [Google Scholar] [CrossRef]
- Lasoń, E. Topical Administration of Terpenes Encapsulated in Nanostructured Lipid-Based Systems. Molecules 2020, 25, 5758. [Google Scholar] [CrossRef] [PubMed]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Bactericidal potentiality of purified terpenoid extracts from the selected sea weeds and its mode of action. J. Trop. Life Sci. 2020, 10, 197–205. [Google Scholar] [CrossRef]
- Gaba, S.; Saini, A.; Singh, G.; Monga, V. An insight into the medicinal attributes of berberine derivatives: A review. Bioorg. Med. Chem. 2021, 38. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2021, 130, 1154–1172. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Zhang, L.; Yang, Y.; Liu, R.; Li, Z. Comparison of Different Chitosan Lipid Nanoparticles for Improved Ophthalmic Tetrandrine Delivery: Formulation, Characterization, Pharmacokinetic and Molecular Dynamics Simulation. J. Pharm. Sci. 2020, 109, 3625–3635. [Google Scholar] [CrossRef]
- Wang, M.; Ma, B.; Ni, Y.; Xue, X.; Li, M.; Meng, J.; Luo, X.; Fang, C.; Hou, Z. Restoration of the Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus aureus and Extended-Spectrum β-Lactamases Escherichia coli through Combination with Chelerythrine. Microb. Drug Resist. 2021, 27, 337–341. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Tragacanth nanocapsules containing Chamomile extract prepared through sono-assisted W/O/W microemulsion and UV cured on cotton fabric. Carbohydr. Polym. 2017, 170, 234–240. [Google Scholar] [CrossRef]
- Lis, M.J.; Carmona, Ó.G.; Carmona, C.G.; Bezerra, F.M. Inclusion complexes of citronella oil with β-cyclodextrin for controlled release in biofunctional textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef] [Green Version]
- Mele, E. Electrospinning of essential oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Cheng, D.; Lu, S.; Huang, F.; Li, G. Preparation of quaternary ammonium salt of chitosan nanoparticles and their textile properties on Antheraea pernyi silk modification. Text. Res. J. 2014, 84, 2115–2124. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Fernandes, M.M.; Mendoza, E.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Sonochemical coating of textiles with hybrid ZnO/chitosan antimicrobial nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 1164–1172. [Google Scholar] [CrossRef]
- Scacchetti, F.A.P.; Pinto, E.; Soares, G.M.B. Thermal and antimicrobial evaluation of cotton functionalized with a chitosan–zeolite composite and microcapsules of phase-change materials. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Yaswant, G.; Kavitha, S.; Chandramohan, E.; Kowsalya, G.; Vijay, R.; Sudhagar, B.; Kumar, D.S.R.S. Preparation and characterization of hybrid chitosan-silver nanoparticles (Chi-Ag NPs); A potential antibacterial agent. Int. J. Biol. Macromol. 2019, 141, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.S.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional chitosan/gold nanoparticles coatings for biomedical textiles. Nanomaterials 2019, 9, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štular, D.; Jerman, I.; Simončič, B.; Tomšič, B. Tailoring of temperature-and pH-responsive cotton fabric with antimicrobial activity: Effect of the concentration of a bio-barrier-forming agent. Carbohydr. Polym. 2017, 174, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Manukumar, H.M.; Umesha, S.; Kumar, H.N.N. Promising biocidal activity of thymol loaded chitosan silver nanoparticles (T-C@AgNPs) as anti-infective agents against perilous pathogens. Int. J. Biol. Macromol. 2017, 102, 1257–1265. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Shaarawy, S.; Mohamed, A.L.; Hebiesh, A. Multifarious cellulosic through innovation of highly sustainable composites based on Moringa and other natural precursors. Int. J. Biol. Macromol. 2020, 165, 141–155. [Google Scholar] [CrossRef]
- Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K.; Ranjithkumar, R.; Bhuvaneshwari, V.; Bharathi, D. Synthesis and characterization of chitosan/zinc oxide nanocomposite for antibacterial activity onto cotton fabrics and dye degradation applications. Int. J. Biol. Macromol. 2020, 164, 2779–2787. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J. Mol. Liq. 2021, 334. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M.; Manikandan, E.; Kumar, S.S.; Pugazhendhi, A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B 2018, 188, 126–134. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L. Nanoparticles as tools to study and control stem cells. J. Cell. Biochem. 2009, 108, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. NBM 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Azevedo, C.; Macedo, M.H.; Sarmento, B. Strategies for the enhanced intracellular delivery of nanomaterials. Drug Discov. Today 2018, 23, 944–959. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro-and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-Da-Silva, F.; Dessi, M.; Gloria, A.; Ambrosio, L.; Gonca̧lves, R.M.; Barbosa, M.A. Layer-by-layer self-assembly of chitosan and poly(γ-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef]
- Oksal, E.; Pangestika, I.; Muhammad, T.S.T.; Mohamad, H.; Amir, H.; Kassim, M.N.I.; Andriani, Y. In vitro and in vivo studies of nanoparticles of chitosan-Pandanus tectorius fruit extract as new alternative treatment for hypercholesterolemia via Scavenger Receptor Class B type 1 pathway. Saudi Pharm. J. 2020, 28, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Onyebuchi, C.; Kavaz, D. Chitosan and N, N, N-trimethyl chitosan nanoparticle encapsulation of ocimum gratissimum essential oil: Optimised synthesis, in vitro release and bioactivity. Int. J. Nanomed. 2019, 14, 7707–7727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.S.; Antunes, J.C.; Homem, N.C.; Felgueiras, H.P. Controlled Release of Cinnamon Leaf Oil from Chitosan Microcapsules Embedded within a Sodium Alginate/Gelatin Hydrogel-Like Film for Pseudomonas aeruginosa Elimination. In Proceedings of the First International Conference on “Green” Polymer Materials 2020, Online, 5–25 November 2020. [Google Scholar]
- Moraes, F.C.; Antunes, J.C.; Forero Ramirez, L.M.; Aprile, P.; Franck, G.; Chauvierre, C.; Chaubet, F.; Letourneur, D. Synthesis of cationic quaternized pullulan derivatives for miRNA delivery. Int. J. Pharm. 2020, 577. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.T.; et al. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 2020, 34. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M.E. Polymeric nanoparticles as oral delivery systems for a grape pomace extract towards the improvement of biological activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef] [PubMed]
- Sahyon, H.A.; Al-Harbi, S.A. Antimicrobial, anticancer and antioxidant activities of nano-heart of Phoenix dactylifera tree extract loaded chitosan nanoparticles: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 160, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, R.; Ardakani, M.T.; Verdom, B.H.; Bagheri, A.; Mohammad-Beigi, H.; Aliakbari, F.; Salehpour, Z.; Alipour, M.; Afrouz, S.; Bardania, H. Chitosan nanoparticles containing Physalis alkekengi-L extract: Preparation, optimization and their antioxidant activity. Bull. Mater. Sci. 2019, 42. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery-In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Wu, K.; Zhou, X.; Ge, F. Loading zedoary oil into pH-sensitive chitosan grafted mesoporous silica nanoparticles via gate-penetration by supercritical CO2 (GPS). J. CO2 Util. 2019, 33, 12–20. [Google Scholar] [CrossRef]
- Manne, A.A.; Arigela, B.; Giduturi, A.K.; Komaravolu, R.K.; Mangamuri, U.; Poda, S. Pterocarpus marsupium Roxburgh heartwood extract/chitosan nanoparticles loaded hydrogel as an innovative wound healing agent in the diabetic rat model. Mater. Today Commun. 2021, 26. [Google Scholar] [CrossRef]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of chitosan nanoparticles and soluplus micelles to optimize the bioactivity of posidonia oceanica extract on human neuroblastoma cell migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: A nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biol. Macromol. 2020, 143, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, N.; Eftekhari, Z.; Roodbari, N.H.; Parivar, K. Evaluation of Encapsulated Eugenol by Chitosan Nanoparticles on the aggressive model of rheumatoid arthritis. Int. Immunopharmacol. 2020, 85. [Google Scholar] [CrossRef] [PubMed]
- Alshubaily, F.A. Enhanced antimycotic activity of nanoconjugates from fungal chitosan and Saussurea costus extract against resistant pathogenic Candida strains. Int. J. Biol. Macromol. 2019, 141, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Sousa, I.M.O.; Queiroz, N.C.A.; Zago, P.M.W.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; San Román, J.; et al. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun biotextiles in tissue engineering and biomolecules delivery systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef]
- Teixeira, M.O.; Antunes, J.C.; Felgueiras, H.P. Recent advances in fiber–hydrogel composites for wound healing and drug delivery systems. Antibiotics 2021, 10, 348. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr. Polym. 2021, 259. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.J.; Burdick, J.A. Advances in nanofibrous scaffolds for biomedical applications: From electrospinning to self-assembly. Nano Today 2014, 9, 722–742. [Google Scholar] [CrossRef]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous materials and their applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Almeida, L.R.; Martins, A.R.; Fernandes, E.M.; Oliveira, M.B.; Correlo, V.M.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Durães, N.F.; Silva, C.J.; et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater. 2013, 9, 8167–8181. [Google Scholar] [CrossRef]
- Morris, H.; Murray, R. Medical textiles. Text. Prog. 2020, 52, 1–127. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-functional textiles: Combining pharmaceutical nanocarriers with fibrous materials for innovative dermatological therapies. Pharmaceutics 2019, 11, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, R.; Radhai, R.; Balakumar, C.; Ahamed, H.A.M.; Vigneswaran, C.; Vaideki, K. Synthesis and characterization of neem chitosan nanocomposites for development of antimicrobial cotton textiles. J. Eng. Fibers Fabr. 2012, 7, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Sagharjoghi Farahani, M.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Amorim, T.M.P. New method to produce poly(vinyl alcohol)/cellulose acetate films with improved antibacterial action. Mater. Today Proc. 2020, 31, S269–S272. [Google Scholar] [CrossRef] [Green Version]
- Sruthi, R.; Balagangadharan, K.; Selvamurugan, N. Polycaprolactone/polyvinylpyrrolidone coaxial electrospun fibers containing veratric acid-loaded chitosan nanoparticles for bone regeneration. Colloids Surf. B 2020, 193. [Google Scholar] [CrossRef] [PubMed]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Oliveira, J.M.; Reis, R.L.; Soria, J.M.; Gómez-Ribelles, J.L.; Mano, J.F. Novel poly(L-lactic acid)/hyaluronic acid macroporous hybrid scaffolds: Characterization and assessment of cytotoxicity. J. Biomed. Mater. Res. A 2010, 94, 856–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Huang, L.; Chen, H.; Qian, M.C.; Ji, H. Pore size matching up: A novel insight into cotton textile aromatic finishing. Flavour Fragr. J. 2020, 35, 149–156. [Google Scholar] [CrossRef]
- Kala, S.; Agarwal, A.; Sogan, N.; Naik, S.N.; Nagpal, B.N.; Patanjali, P.K.; Kumar, J. Chitosan-acrylate nanogel for durable anti mosquito finishing of cotton fabric and its dermal toxicity profiling on Swiss albino mice. Colloids Surf. B 2019, 181, 789–797. [Google Scholar] [CrossRef]
- Subramani, K.; Shanmugam, B.K.; Rangaraj, S.; Palanisamy, M.; Periasamy, P.; Venkatachalam, R. Screening the UV-blocking and antimicrobial properties of herbal nanoparticles prepared from Aloe vera leaves for textile applications. IET Nanobiotechnol. 2018, 12, 459–465. [Google Scholar] [CrossRef]
- Ali, N.F.; Abd-Elsalam, I.S. Antimicrobial characteristics of wool fibers treated with chitosan-propolis nano composite and dyed with natural dye extracted from Red Prickly Pear. Int. J. Agric. Technol. 2020, 16, 223–236. [Google Scholar]
- Hu, J.; Xiao, Z.B.; Zhou, R.J.; Ma, S.S.; Li, Z.; Wang, M.X. Comparison of compounded fragrance and chitosan nanoparticles loaded with fragrance applied in cotton fabrics. Text. Res. J. 2011, 81, 2056–2064. [Google Scholar] [CrossRef]
- Rajendran, R.; Radhai, R.; Kotresh, T.M.; Csiszar, E. Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydr. Polym. 2013, 91, 613–617. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Liu, Y.; Chen, M.; Hu, Y.; Yang, Z. Study on the grafting of chitosan-gelatin microcapsules onto cotton fabrics and its antibacterial effect. Colloids Surf. B 2013, 109, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.C.L.; Wang, W.Y.; Kan, C.W.; Ng, F.S.F.; Wat, E.; Zhang, V.X.; Chan, C.L.; Lau, C.B.S.; Leung, P.C. Microencapsulation of Traditional Chinese Herbs-PentaHerbs extracts and potential application in healthcare textiles. Colloids Surf. B 2013, 111, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, Z.; Xiao, Z.; Ji, H. Preparation and controllable release of chitosan/vanillin microcapsules and their application to cotton fabric. Flavour Fragr. J. 2014, 29, 114–120. [Google Scholar] [CrossRef]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D.; Molina, J.; Souto, A.P.; Fangueiro, R.; Zille, A. Properties and controlled release of chitosan microencapsulated limonene oil. Rev. Bras. Farmacogn. 2014, 24, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Damani, M.; Baxi, K.; Aranha, C.; Sawarkar, S.P. Recent advances in herbal drug nanocarriers against cervical cancer. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 37–78. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, S.; Yadav, J.S. A review on recent trends in green synthesis of gold nanoparticles for tuberculosis. Adv. Pharm. Bull. 2021, 11, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sheikh, J.; Annu; Singh, A. An eco-friendly route to develop cellulose-based multifunctional finished linen fabric using ZnO NPs and CS network. J. Ind. Eng. Chem. 2021, 97, 383–389. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. extract. Carbohydr. Polym. 2021, 256. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Sisubalan, N.; Sridevi, M.; Varaprasad, K.; Ghouse Basha, M.H.; Shucai, W.; Sadiku, R. Biocidal chitosan-magnesium oxide nanoparticles via a green precipitation process. J. Hazard. Mater. 2021, 411. [Google Scholar] [CrossRef]
- Mbae, K.M.; Umesha, S. Physicochemical and antimicrobial properties of post-synthesis betanin and chitosan oligosaccharide functionalized silver nanoparticles. J. Nanopart. Res. 2020, 22. [Google Scholar] [CrossRef]
- Mughees, M.; Wajid, S. Herbal based polymeric nanoparticles as a therapeutic remedy for breast cancer. Anti Cancer Agents Med. Chem. 2021, 21, 433–444. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Nzila, A.; Obot, I.B. Preparation of silver/chitosan nanofluids using selected plant extracts: Characterization and antimicrobial studies against gram-positive and gram-negative bacteria. Materials 2020, 13, 1629. [Google Scholar] [CrossRef] [Green Version]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236. [Google Scholar] [CrossRef]
- Kamel, R.; Salama, A.; Shaffie, N.M.; Salah, N.M. Cerebral effect of optimized Allium sativum oil-loaded chitosan nanorods: GC-MS analysis and in vitro/in vivo evaluation. Food Funct. 2020, 11, 5357–5376. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.S.; Oshiro-Junior, J.A.; Sato, M.R.; Conceição, M.M.; Medeiros, A.C.D. Polymeric nanoparticle associated with ceftriaxone and extract of schinopsis brasiliensis engler against Multiresistant enterobacteria. Pharmaceutics 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.D.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertodi, F.C.; et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef] [PubMed]



| CS or CS Derivative | CS-Based Structure(s) | AM Features | Afflicted Bacteria | Intended Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| DA | Derivative | DS | Mw | |||||
| 23–62% | Thymine-modified CS | - | 154–194 kDa | CS porous sponges | Wrinkled and damaged cell walls, particularly with increased DS, which increased CS’ solubility and charge density. 100% cell death. | Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii | Wound dressing | [31] |
| - | - | - | 50–190 kDa | Core [gelatin (GN) + poly(vinylpolypyrrolidone) (PVP) + imipenem/cilastatin]—shell (CS + poly(ethylene oxide) (PEO) + vancomycin) nanofibers | Zone of inhibition (ZoI) of 2.45, 2.90, 2.75, and 1.85 cm, respectively. CS enabled controlled release of the antibody for higher global efficiency. | MRSA, S. aureus, P. Aeruginosa, and E. coli | Wound dressing | [32] |
| ≥10% | Carboxymethyl CS | ≥20% | 10–20 kDa | Carboxymethyl CS loaded with waterborne polyurethane–GN hydrolysate hydrogel film | ZoI of 12–16 and 16–20 mm, respectively. Higher activity of higher CS derivative amount, especially at lower pH. | S. aureus and E. coli | Wound dressing | [33] |
| 9.7% | - | - | 100–300 kDa | Cinnamon leaf or clove-oil-loaded CS and poly(vinyl alcohol) (PVA) blended films | CS films alone were effective against both bacteria and capable of eradicating all P. aeruginosa in 1 h (*** p < 0.001). Still, loaded CS/PVA films showed significantly improved AM traits in relation to unloaded films within 2 h of contact. | S. aureus and P. aeruginosa | Wound dressing | [34] |
| 15% | - | - | Low | Thyme-oil-loaded CS-tripolyphosphate (TPP) microcapsules spray dried onto linen fabric | >98% growth inhibition due to oil and CS joint action. | E. coli | Textile finishing | [35] |
| 26% | - | - | 292 kDa | TiO2 nanoparticles (NPs) dispersed onto CS–glycerol-coated cotton fabric | 99.8 and 97.3% bacterial reduction, respectively, driven by CS’s cationic nature. | S. aureus and E. coli | Textile finishing | [36] |
| 15% | Quaternized tosyl CS | 45–55% | - | Crosslinked hydrogels of CS derivative and GN | Quaternary CS (replacing primary -OH) and free amino groups interacted with the anionic bacterial membrane, and the lipophilic chain perturbed the hydrophobic domains of the cell envelope. Minimum inhibitory concentration (MIC): 128−256, 64−128, 256, 256−512, 64 to 128, 64−128, 64−256, and 256−512 μg/mL, respectively. | MRSA, S. epidermidis, P. aeruginosa, A. baumannii, vancomycin-resistant S. aureus (VRSA), E. faecium, vancomycin-resistant Enterococcus (VRE) and E. coli | Healthcare infection control | [37] |
| 15% | - | - | Medium | TPP-crosslinked CS, GN, potato-starch, and banana peel powder (BPP) blended films | ZoI of 5–8 (S. aureus) and 9–11 mm (E. coli), because of the CS and BPP combined effect. | S. aureus and E. coli | Wound dressings | [38] |
| 29% | - | - | - | CS-coated UV-disinfected Vicryl sutures | Growth inhibition of both bacterial and fungal pathogens, profound inhibition of slime formation, and mixed-species biofilm inhibition, as a result of CS’s activity. No hyphal formation. | S. epidermidis and Candida albicans | Surgical sutures | [39] |
| 10% | O-carboxymethyl CS | 80% | 200 kDa | O-carboxymethyl CS and Jeffamine porous hydrogel | ≥99% bacterial reduction on account of CS’s amine groups. | E. coli | Wound dressing, drug delivery, and tissue engineering | [40] |
| - | Methacrylated glycol CS | 70% | - | β-cyclodextrin-/triclosan-complex-grafted methacrylated glycol CS | Full inhibition of bacterial infection in 5 h and improved wound healing, attributed to the hydrophilic/hydrophobic nature of CS derivative. | S. aureus and E. coli | Tissue adhesives for wound closure | [41] |
| - | Quaternized CS | 26% | - | Crosslinked (carbodiimide chemistry) quaternized CS-coated titanium printed cages | ZoI: 15 mm2, 0 CFU/mL, decreased crystal violet staining, in vivo inhibition of bacterial growth throughout the entire observation period (1–5 d), and reduced bacterial quantity in the extracted cages. | S. aureus | Intervertebral fusion cages | [42] |
| 15% | - | - | 5–20 mPa.s | Spray-dried CBO-loaded CS and gelatin microcapsules | Over 90% growth inhibition until 10 fabric washes. | S. aureus and E. coli | Functional finishing of linen | [43] |
| - | - | - | Medium | Self-assembled nanogels of gluthathione–silver (Ag) nanoclusters (NCs) and CS | Improved antibacterial action (>10-fold), with the well-dispersion of the ultrasmall Ag NCs in the CS framework protecting Ag NCs from decomposition and aggregation and allowing a slow release of Ag+ ions; the positively charged CS carrier substantially promotes Ag–bacteria interaction and the concomitant Ag bactericidal activity. | S. aureus, E. coli, Bacillus subtilis, and P. aeruginosa | Theranostic nanomedicines | [44] |
| 21% | - | - | Medium | CS-TPP NPs incorporated within cotton fabric via pad-dry-curing | Increased ZoI: ≈20 (S. aureus and B. subtilis), ≈16 (E. coli and Proteus vulgaris), ≈25 mm (C. albicans and A. Niger) due to CS-based NP action. | S. aureus, B. subtilis, E. coli, Proteus vulgaris, C. albicans and Aspergillus Niger | Textile finishing | [45] |
| - | - | - | - | CS-coated PCl microparticles (MPs) encapsulating Ag NPs, then entrapped into PVA/PVP microneedle layers | pH-triggered Ag release enabled 100% eradication of bacterial bioburdens from an ex vivo biofilm model in rat skin, given the feasibility of the loading of silver NPs into responsive MPs. | S. aureus and P. aeruginosa | Biofilm skin infections | [46] |
| - | - | - | - | CS hydrogel combined with zinc oxide/zeolite nanocomposite | 33 and 45% biofilm formation and metabolic activity reduction, due to a joint effect from the nanocomposite’s elements. Significantly decreased gtfB, gtfC, and ftf reinforcing lower bacterial adhesion. | Streptococcus mutans | Dental biofilm control | [47] |
| - | Double bond modified N-dodecylated CS | - | - | Macroporous cryogel containing double bond modified N-dodecylated CS and graphene oxide (GO) | Excellent near-infrared (NIR)-assisted photothermal antibacterial activity against both bacteria and killed 99% of them after 20 min NIR irradiation, because of the CS derivative and GO. | S. aureus and E. coli | Clinical hemorrhage and infection control | [48] |
| 5–10% | Quaternary CS | 76.4% | 340 kDa | Quaternary CS/PVA nanofiber membrane crosslinked with blocked diisocyanate | ~100% antibacterial efficacy, attributed to the CS derivative permanently cationic net charge. | E. coli | Wound dressings | [49] |
| <25% | - | - | 310–375 kDa | Graphene/CS/magnetite NPs | ZoI of 21.3 and 19.3 mm and MIC of 60 and 70 μg/mL, respectively, due to synergistic antibacterial action of NP constituents. | ESBL-producing P. aeruginosa and Klebsiella pneumoniae | Biomedical applications with antibacterial requirement | [50] |
| - | N–succinyl CS | - | 150 kDa | PVA/N–succinyl CS/lincomycin porous hydrogels | ~100% and ~70% antibacterial efficacy, respectively, with the antibiotic being held responsible for most of it. | S. aureus and E. coli | Wound dressings | [51] |
| 15–25% | - | - | Medium | Ag NP-doped multilayered CS hydrogel | ZoI: ~7 and ~12 mm, promoted by staged release pattern of Ag NPs based on acid triggered dissolution of the multi-membrane layer by layer. | S. aureus and E. coli | Implant coating or wound dressings | [52] |
| - | Quaternized CS | - | - | Double-crosslinked oxidized dextran–dopamine and quaternary CS with encapsulated Ag NPs and deferoxamine | 15.5 and 20.8% survival rate, plus 3/97% and 9/91% live/dead cells, respectively, through the combination of Ag NPs and HTCC. | S. aureus and E. coli | Bacterial infected diabetic wound dressing | [53] |
| - | Quaternized CS | 22% | - | Protocatechuic-acid-grafted quaternized CS | Excellent antibacterial properties and showed a satisfactory synergistic antibacterial effect with protocatechuic acid. | S. aureus and MRSA | Infection control | [54] |
| 15–25% | - | - | 50–190 kDa | Tea-tree-oil-loaded CS-poly(ε-caprolactone) core-shell nanocapsules | Increased cell death (17%), following contact with released essential oil and CS shell. | Cutibacterium acnes | Topical acne treatment | [55] |
| - | - | - | - | Thiolated CS/Ag nanowire composite hydrogels | Increased ZoI because of CS derivative and Ag joint action. | S. aureus and E. coli | Obstetric wound care | [56] |
| 8% | Fluorinated quaternary CS | - | 50–190 kDa | Fluorinated quaternary CS | Bacterial cell death in 6 h. MICs of 64 to 512 μg/mL (Gram-positive bacteria) and 128 to 512 (Gram-negative bacteria), particularly effective against MRSA and B. subtilis. Fluorination and quaternization of CS improved its solubility and antimicrobial activity. Fluorine is the most electronegative element with a strong effect on the conformational and physicochemical properties of organic compounds. | MRSA, E. coli, P. aeruginosa, Streptococcus sanguinis, Salmonella enterica, S. epidermidis, B. subtilis, and S. aureus | Infection control | [57] |
| 15–25% | Mannose-functionalized CS | - | Medium | Mannose-functionalized CS nanosystems | Particular bacterial growth inhibition (4× lower), anti-adherence (4× lower), and biofilm disruption (3–6× lower) activity. Electrostatic interaction disturbed the bacterial membrane integrity, osmolarity, and depletion of nutrients. With mannose, it interacted with bacterial membrane lectins, interfering with adhesion and motility. | Multidrug-resistant clinical isolates of E. coli, Listeria monocytogenes, S. aureus, and P. aeruginosa | Infection control | [58] |
| 15% | N-halamine hydantoin-containing CS | 56% | 250 kDa | N-halamine hydantoin-containing CS films | 0.003% and 0.218% CFU/mL, on account of the biocidal N–Cl bonds added to the already antibacterial CS. | S. aureus and E. coli | Infection control | [59] |
| 15–25% | - | - | 100–300 kDa | CS–hyaluronic acid polyelectrolyte multilayered coating of nylon monofilament sutures | Significant growth inhibition in the first hours of contact, given antibacterial features of the built coating. | S. aureus and E. coli | Sutures | [60] |
| 15–25% | Catechol-modified quaternized CS | Medium | Catechol modified quaternized CS incorporated into PDLLA-PEG-PDLLA hydrogel | >95% bacterial cell death, potentiated by the quaternized CS moieties. | S. aureus and E. coli | Wound dressings | [61] | |
| - | - | - | - | Cellulose acetate nanofibers coated with CS nanowhiskers | 99% growth inhibition due to CS nanowhisker activity. | E. coli | Biomedical applications with antibacterial requirement | [62] |
| - | N-succinyl CS | - | Low | N-succinyl CS-ZnO NPs conjugated with curcumin | MIC reduction of 25-to-50-fold and minimum bactericidal concentration (MBC) reduction of 10-to-40-fold, respectively, given curcumin addition to NPs containing CS derivative and ZnO, all endowed with antibacterial traits. | S. aureus and E. coli | Biomedical applications with antibacterial requirement | [63] |
| - | - | - | Medium | CS and β-glycerolphosphate hydrogel | In vitro unresponsiveness but clear in vivo bacterial reduction, as treated wounds were completely re-epithelialized and closed on day 14 post-surgery. | A. baumannii | Wound dressings | [64] |
| Antibacterial Compound Classes | Description | Examples | Ref. | ||
|---|---|---|---|---|---|
| Phenols and polyphenols | Simple phenols | Single substituted phenolic ring | Eugenol | Thymol | [34,54,70,87,91,92,93] |
| Phenolic acids | C6-C1 (hydroxybenzoic acids) or C6-C3 (hydroxycinnamic acids), consisting of a phenolic ring and a carboxyl substituent | Gallic acid | Protocatechuic acid | [34,54,70,87,91,92,93] | |
| Quinones | Aromatic rings with two carbonyl groups | Thymoquinone | 1,4-Naphthoquinone | [94,95,96,97,98,99,100,101] | |
| Flavonoids | Phenolic compounds that include a C6-C3-C6 carbon framework (phenyl benzopyran) | Quercetin | Vaccarin | [102,103,104,105,106,107] | |
| Tannins | Hydrolysable tannins: central core of glucose or another polyol esterified with gallic acid, also called gallotannins, or with hexahydroxydiphenic acid, also called ellagitannins; condensed tannins: oligomers or polymers composed of flavan-3-ol nuclei. | Gallocatechin | (−)-Epigallocatechin gallate | [14,108,109] | |
| Coumarins | Phenolic substances with fused benzene and α-pyrone rings | Novobiocin | Chlorobiocin | [77,78,79,80] | |
| Terpenes and terpenoids | General chemical structure is C10H16, and they occur as diterpenes, triterpenes, and tetraterpenes (C20, C30, and C40), hemiterpenes (C5) and sesquiterpenes (C15). In terpenoids methyl groups are moved/removed, or functional groups (usually oxygen-containing) are added. | Limonene | Geraniol | [81,110,111,112,113,114,115] | |
| Alkaloids | Heterocyclic nitrogen compounds | Tetrandrine | Berberine | [116,117,118,119,120] | |
| CS or CS Derivative | Carrier Composition | Production Method | Loaded Plant Extract | Main Particle Features | Main Observed Effects | Potential Applications | Appointed Release Mechanism | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | Derivative | DS | Mw | Hydrophilic | Hydrophobic | |||||||
| 15 | - | - | 60 kDa | CS/poly(γ-glutamic acid) (γ-PGA) | Polyelectrolyte complexation | - | Tea catechins | Round-shaped; dDLS = 134–147 nm; ζ = −18.7–33.5 mV (Δ molar ratio) | Enhanced antioxidant activity. | Dietary supplements | pH-triggered disintegration | [14] |
| - | - | - | Low | CS/TPP | Ionic gelation | - | Grape pomace extract | Round-shaped; dDLS = 419–853 nm; ζ = 7.4–14.9 mV (Δ CS and drug concentration) | High antioxidant capacity and antimicrobial action against methicillin-susceptible S. aureus, L. monocytogenes, P. aeruginosa, S. enteritidis, E. coli, and C. albicans. Reduced in vitro intestinal permeability. | Dietary supplements | pH-triggered release | [157] |
| - | - | - | 100–200 kDa | CS/TPP | Ionic gelation | P. dactylifera extract | - | Round-shaped; dDLS ≈ 210 nm; ζ = 33 mV | Antioxidant, antibacterial, antifungal, and anticancer (yet protecting vital organs from oxidative stress). | Dietary supplements | - | [158] |
| 15–25% | - | - | - | CS/lecithin | Nanoprecipitation or solvent displacement | - | Thyme | Round-shaped; dTEM = 6.4 (NPs) or 9.1 nm (nanocapsules) | Controllable release kinetics with significant inhibitory effects against S. aureus and Bacillus cereus. | Antimicrobial medication against foodborne bacteria | Desorption, or diffusion | [88] |
| - | - | - | 190–310 kDa | CS/fucoidan | Polyelectrolyte complexation | - | Quercetin | dDLS = 356 nm; ζ = −30 mV | Controlled release under biorelevant simulated gastrointestinal environments; significant antioxidant activity. | Nutraceutical and pharmaceutical uses | pH-responsive diffusion, combined with carrier erosion | [159] |
| 15–25% | - | - | Medium | CS/TPP/sodium hexametaphosphte (HMP) | Emulsification and ionic gelation | - | Carum copticum | Round-shaped; dDLS = 236.0–721.0 nm | Improved antimicrobial and antioxidant effects. | Nutraceutical, cosmetic and pharmaceutical uses | Desorption, then diffusion, especially in alkaline conditions | [149] |
| 15.2% | - | - | Medium | CS/TPP/Tween 80 | Emulsification followed by ionic gelation | - | Peppermint and green tea oils | Round-shaped; dTEM = 20–60 nm, dDLS = 252.6–256.3 nm; ζ = −20.9–29.0 mV (Δ molar ratio) | Increased thermal stability; enhanced antioxidant activity and antimicrobial action. | Nutraceuticals, cosmetic and pharmaceutical uses. | Diffusion | [75] |
| - | - | - | Low | CS/TPP | Ionic gelation | - | Physalis alkekengi-L | Round-shaped; dSEM = ~160 nm, dDLS = 196 nm; ζ= 7.69 mV | Prolonged antioxidant activity with potential anticancer performance. | Antioxidant medical formulations | Diffusion | [160] |
| 4% | - | - | Medium | CS/polysorbate 80 | Ionic gelation | - | Thymoquinone | Round-shaped; dTEM = 74.66 nm, dDLS = 492.3 nm; ζ= 3.89 mV | Elevated monoamine neurotransmitter synthesis, particularly serotonin, and prevented oxidative stress on neural cells (enhanced antidepressant effects). | Antidepressants for mental illnesses | Desorption, then diffusion | [76] |
| 14% | - | - | ~50 kDa | CS/TPP | Ionic gelation | - | Rosmarinic acid, Salvia officinalis (sage) and Satureja montana (savory) | Round-shaped; dDLS = 280.0–302.4 nm; ζ= 27.5–30.1 mV | Increased permeability and retention; no cytotoxic effects. | Treatment of oxidative eye conditions | pH-triggered disintegration | [161] |
| - | - | - | - | Carboxymethyl CS, hydroxypropyl CS or trimethyl CS/poloxamer 407/Kolliphor® HS 15 | Emulsification | - | Tetrandrine | Round-shaped; dDLS = 157.0 nm; ζ = 22.1 mV | Improved drug sustained release and bioavailability; no sign of ocular irritation. | Treatment of glaucoma | Desorption, then diffusion | [119] |
| ≤25% | - | - | - | CS/alginate/tween 80/CaCl2 | Emulsification and ionic gelation | - | Turmeric and lemongrass oil | Round-shaped; dDLS = 226.4–256.6 nm; ζ = 35.7–37.3 mV | Hemocompatible, nontoxic systems with a sustained drug release profile; antibacterial, antifungal, antioxidant, antimutagenic, and anticarcinogenic properties. | Medical and pharmaceutical drug delivery systems | pH-responsive diffusion | [73] |
| 10 | - | - | 150 kDa | Citric acid-CS/TPP and N, N, N-trimethyl CS/TPP | Emulsification and ionic gelation | - | Ocimum gratissimum essential oil | Round-shaped; dDLS = 134.9 and 153.5 nm; ζ = 26.1 and 22.6 mV, respectively | Increasing antioxidant activity even after 75 h. With, CS derivative, antibacterial activity at a lower concentration for both Gram-negative and Gram-positive food pathogens. Toxic towards MDA-MB-231 breast cancer cell lines. | Antioxidant, antibacterial and anticancer agents | Desorption, then diffusion | [151] |
| - | - | - | - | CS grafted to mesoporous silica NPs | Emulsification, chemical grafting and gate-penetration by super-critical CO2 | - | Zedoary oil | Mesoporous round-shaped; dDLS = 86.7 nm | Controlled release triggered by pH changes; increased stability of the loaded molecule | Drug delivery systems | pH-responsive diffusion | [162] |
| ≤15% | - | - | Medium | CS/Pterocarpus marsupium | Ionic gelation | Pterocarpus marsupium | - | Round-shaped; dDLS = 676 nm; ζ = 57.3 mV | Higher stability, enhanced entrapment efficiency, and sustained drug release characteristics. Significant increase in alpha-amylase inhibition and appreciable anti-inflammatory activity. | Therapeutic agent against diabetes and inflammatory disorders in drug delivery applications | Desorption, then slow degradation and diffusion | [163] |
| 15% | - | - | 50–190 kDa | CS/quinoline/Tween 60 | Nanoemulsion | - | Quercetin | Nanorod shape and monolithic structure; dDLS = 141–174.8 nm; ζ = −2.4 to −14.1 mV | Enhanced pH-sensitive controlled release; remarkable anticancer activity against HeLa cells by reducing cancer cells’ proliferative skills. | Anticancer drug nanocarriers | pH-responsive diffusion | [74] |
| 15–25% | - | - | 50–190 kDa | CS/TPP | Ionic gelation | - | Posidonia oceanica | Round-shaped; dDLS = 252.4 nm; ζ= 19.7 mV | Excellent physical and chemical stability during storage; enhanced extract solubility and prolonged release; improved inhibitory effect on cell migration. | Treatments to prevent neuroblastoma cell migration | Diffusion | [164] |
| - | - | - | - | CS/Tween 20 | Nanoemulsification | - | Zataria multiflora oil | Round-shaped; dDLS = 463 nm; ζ = 18.35 mV | Improved the proliferation inhibition rate of breast cancer cells by inducing apoptosis, generating ROS, and triggering mitochondrial membrane permeabilization, while damaging cell DNA without harming normal cells. | Breast cancer medication | - | [165] |
| ≤10% | - | - | 50–190 kDa | CS/Liquid paraffin/Tween 80/Span 80/magnesium stearate | Emulsification | - | Cinnamaldehyde | Round-shaped; dTEM = 80–150 nm | Increased chemical stability and synergistic antibacterial action against Gram-positive and Gram-negative bacteria. | Medical textiles (e.g., wound dressings) | - | [89] |
| 5% | - | - | - | CS/TPP | Ionic gelation | - | Vaccarin | Round-shaped; dTEM ≈ 40 nm, dDLS = 216.6 nm; ζ = 37.1 mV | No evidence of cytotoxic effects; increased umbilical vein endothelial cells proliferation and migration; up-regulated IL-1β and PDGF-BB factors, promoting angiogenesis. | Wound healing | Burst, then sustained release | [105] |
| ≤15% | - | - | - | CS/TPP | Ionic gelation | Pterocarpus marsupium Roxburgh heartwood extract | - | Round-shaped; dSEM = 400 nm; dDLS = 676 nm | Inhibition against Gram-positive and Gram-negative bacteria. Healing of complicated surgical wounds. | Wound healing | Diffusion | [163] |
| 15% | - | - | Low | CS/TPP | Ionic gelation | - | Gallic acid | Round-shaped; dDLS = 117.5–356.6 nm; ζ = 18.3–33.6 mV | Accelerates angiogenesis, hexosamine synthesis, collagen deposition, and recruiting immune cells at wound area. | Wound healing dressings | pH-triggered desorption, then disintegration | [87] |
| - | - | - | - | CS/TPP/Tween 80 | Ionic gelation and emulsification | - | Pandanus tectorius fruit extract | Round-shaped; dDLS = 160.4 nm | No evidence of cytotoxic responses; increased SR-B1 gene expression required for an effective reduction of hypercholesterolemia-related symptoms. | Control medication for hypercholesterolemia | - | [150] |
| - | - | - | - | CS/TPP | Ionic gelation | - | Eugenol | Round-shaped; dSEM = 23–16-37.67 nm; ζ = −49.6 mV | Reduced expression of TGF-β and MCP-1 genes; NPs revealed increased immunomodulatory, anti-inflammatory, and antioxidant potential. | Treatment of autoimmune diseases, such as rheumatoid arthritis | - | [166] |
| 21% | - | - | 206.4 kDa | CS/Tween 80 | Emulsification and spray-drying | - | Lemongrass essential oil (LEO) and geranium essential oil (GEO) | Round-shaped; dSEM = 4.959 and 5.009 µm; ζ = 45.26 and 47.34 mV, respectively | Higher thermal and colloidal stability than raw CS and EOs. The MIC for C. albicans was reduced up to 64 times. Reduced biomass of mature biofilm up to 84%. | Compounds that have antibiofilm activity against C. albicans. | Diffusion | [72] |
| 91.2% | - | - | 106.8 kDa | CS/TPP | Ionic gelation | Saussurea costus | - | Round-shaped; dTEM = 48 nm; ζ = 3.28 mV | Notable antimycotic potentiality against all examined strains, with vigorous structural and morphological alterations. | Antimycotic agent to control resistant pathogenic yeast strains | - | [167] |
| 9.6 | - | - | 100–300 kDa | CS/TPP | Ionic gelation | - | Cinnamon leaf oil | Round-shaped | Significant reduction of viable cells, right after 2 h of incubation. | P. aeruginosa’s infection control | pH-responsive release | [152] |
| 17% | - | - | >150 kDa | CS/TPP | Ionic gelation | Arrabidaea chica extract | - | Round-shaped; dTEM = 20–60 nm, dDLS = 60–153 nm; ζ = 32.1–32.9 mV (Δ load content) | Gastroprotective effect. Biocompatibility, antiulcerogenic activity. | Ulcer-healing pharmaceutical systems | - | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Mar. Drugs 2021, 19, 359. https://doi.org/10.3390/md19070359
Antunes JC, Domingues JM, Miranda CS, Silva AFG, Homem NC, Amorim MTP, Felgueiras HP. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Marine Drugs. 2021; 19(7):359. https://doi.org/10.3390/md19070359
Chicago/Turabian StyleAntunes, Joana C., Joana M. Domingues, Catarina S. Miranda, A. Francisca G. Silva, Natália C. Homem, M. Teresa P. Amorim, and Helena P. Felgueiras. 2021. "Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems" Marine Drugs 19, no. 7: 359. https://doi.org/10.3390/md19070359