Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a Tetrahydrooxazolo[3,2-a]azepine-2,5(3H,6H)-dione Backbone from the Red Sea Sponge Negombata magnifica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of 1-5
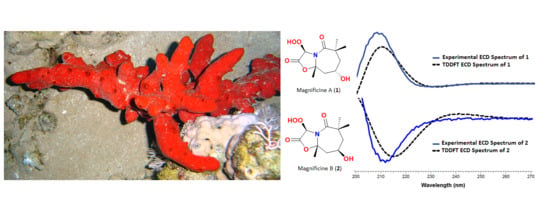
2.2. Structure of Magnificine A (1)
2.3. Structure of Magnificine B (2)
2.4. Structure of (±)-Negombaionone (3)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Purification of Compounds 1-5
3.4. Spectral Data of the Compounds
3.5. Computational Details
3.6. Disk Diffusion Assay
3.7. MIC of the Compounds
3.8. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, M.; Sammaai, T. Family Podospongiidae De Laubenfels. In Systema Porifera: A Guide to Classification of Sponges; Hooper, N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum: New York, NY, USA, 2002; Volume 1, pp. 698–699. [Google Scholar]
- Kashman, Y.; Groweiss, A.; Shmueli, U. Latrunculin, a new 2-thiazolidinone macrolide from the marine sponge Latrunculia magnifica. Tetrahedron Lett. 1980, 21, 3629–3632. [Google Scholar] [CrossRef]
- Groweiss, A.; Shmueli, U.; Kashman, Y. Marine toxins of Latrunculia magnifica. J. Org. Chem. 1983, 48, 3512–3516. [Google Scholar] [CrossRef]
- Blasberger, D.; Carmely, S.; Cojocarub, M.; Ilan Spector, I.; Shochet, N.R.; Kashman, Y. On the chemistry of latrunculins A and B. Liebigs Ann. Chem. 1989, 1171–1188. [Google Scholar] [CrossRef]
- Hoye, T.R.; Ayyad, S.N.; Eklov, B.M.; Hashish, N.E.; Shier, W.T.; El Sayed, K.A.; Hamann, M.T. Toward computing relative configurations: 16-epi-latrunculin B, a new stereoisomer of the actin polymerization inhibitor latrunculin B. J. Am. Chem. Soc. 2002, 124, 7405–7410. [Google Scholar] [CrossRef]
- Vilozny, B.; Amagata, T.; Mooberry, S.L.; Crews, P. A new dimension to the biosynthetic products isolated from the sponge Negombata magnifica. J. Nat. Prod. 2004, 67, 1055–1057. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Youssef, D.T.A.; Marchetti, D. Bioactive natural and semisynthetic latrunculins. J. Nat. Prod. 2006, 69, 219–223. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Odde, S.; Daga, P.R.; Bowling, J.J.; Mesbah, M.K.; Youssef, D.T.; Khalifa, S.I.; Doerksen, R.J.; Hamann, M.T. Latrunculin with a highly oxidized thiazolidinone ring: Structure assignment and actin docking. Org. Lett. 2007, 9, 4773–4776. [Google Scholar] [CrossRef] [Green Version]
- Amagata, T.; Johnson, T.A.; Cichewicz, R.H.; Tenney, K.; Mooberry, S.L.; Media, J.; Edelstein, M.; Frederick, A.; Valeriote, F.A.; Crews, P. Interrogating the bioactive pharmacophore of the latrunculin chemotype by investigating the metabolites of two taxonomically unrelated sponges. J. Med. Chem. 2008, 51, 7234–7242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, N.B.; Blunt, J.W.; McCombs, J.D.; Munro, M.H.G. Discorhabdin C, a highly cytotoxic pigment from a sponge of the genus Latrunculia. J. Org. Chem. 1986, 51, 5476–5478. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G.; Higa, T.; Sakai, R. Discorhabdin D, an antitumor alkaloid from the sponges Latrunculia brevis and Prianos sp. J. Org. Chem. 1988, 53, 4127–4128. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G. Cytotoxic pigments from New Zealand sponges of the genus Latrunculia: Discorhabdins a, b and c. Tetrahedron 1988, 44, 1727–1734. [Google Scholar] [CrossRef]
- Yang, A.; Baker, B.J.; Grimwade, J.; Leonard, A.; McClintock, J.B. Discorhabdin alkaloids from the Antarctic sponge Latrunculia apicalis. J. Nat. Prod. 1995, 58, 1596–1599. [Google Scholar] [CrossRef]
- Dijoux, M.-G.; Gamble, W.R.; Hallock, Y.F.; Cardellina, J.H., II; Van Soest, R.; Boyd, M.R. A new discorhabdin from two sponge genera. J. Nat. Prod. 1999, 62, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hamann, M.T. Atkamine: A new pyrroloiminoquinone scaffold from the cold water Aleutian Islands Latrunculia sponge. Org. Lett. 2013, 15, 1516–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Pandey, P.; Janussen, D.; Chittiboyina, A.G.; Ferreira, D.; Tasdemir, D. Tridiscorhabdin and didiscorhabdin, the first discorhabdin oligomers linked with a direct C–N bridge from the sponge Latrunculia biformis collected from the deep sea in Antarctica. J. Nat. Prod. 2020, 83, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Capon, R.J.; MacLeod, J.K.; Willis, A.C. Trunculins A and B, norsesterterpene cyclic peroxides from a marine sponge, Latrunculia brevis. J. Org. Chem. 1987, 52, 339–342. [Google Scholar] [CrossRef]
- Chao, C.-H.; Chou, K.-J.; Wang, G.-H.; Wu, Y.-C.; Wang, L.-H.; Chen, J.-P.; Sheu, J.-H.; Sung, P.-J. Norterpenoids and related peroxides from the Formosan marine sponge Negombata corticata. J. Nat. Prod. 2010, 73, 1538–1543. [Google Scholar] [CrossRef]
- Řezanka, T.; Dembitsky, V.M. Ten-membered substituted cyclic 2-oxecnone (decalactone) derivatives from Latrunculia corticata, a Red Sea sponge. Eur. J. Org. Chem 2003, 2003, 2144–2152. [Google Scholar] [CrossRef]
- Rudi, A.; Yehuda Benayahu, Y.; Kashman, Y. Negombins A−I, New chlorinated polyfunctional diterpenoids from the marine sponge Negombata species. Org. Lett. 2007, 9, 2337–2340. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mesbah, M.; Youssef, D.; Khalifa, S. Chemical and biological investigation of the Red Sea sponge Negombata corticata. Bull. Pharm. Sci. Assiut 2006, 29, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.A.; Khalifa, S.I.; Hamann, M.T. Antiepileptic ceramides from the Red Sea sponge Negombata corticata. J. Nat. Prod. 2008, 71, 513–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampella, A.; Randazzo, A.; Borbone, N.; Luciani, S.; Trevisi, L.; Debitusc, C.; D’Auria, M.V. Isolation of callipeltins A–C and of two new open-chain derivatives of callipeltin A from the marine sponge Latrunculia sp. A revision of the stereostructure of callipeltins. Tetrahedron Lett. 2002, 43, 6163–6166. [Google Scholar] [CrossRef]
- Sepe, V.; D’Orsi, R.; Borbone, N.; D’Auria, M.V.; Bifulco, G.; Monti, M.C.; Cataniac, A.; Zampella, A. Callipeltins F–I: New antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron 2006, 62, 833–840. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Sepe, V.; Rosa D’Orsi, R.; Bellotta, F.; Debitus, C.; Zampella, A. Isolation and structural elucidation of callipeltins J–M: Antifungal peptides from the marine sponge Latrunculia sp. Tetrahedron 2007, 63, 131–140. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic psammaplysin analogues from the Verongid Red Sea sponge Aplysinella species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Harakeh, S.M.; Genta-Jouve, G. Bioactive diketopiperazines and nucleoside derivatives from a sponge-derived Streptomyces species. Mar. Drugs 2019, 17, 584. [Google Scholar]
- Shaala, L.A.; Youssef, D.T.A.; Alzughaibi, T.A.; Elhady, S.S. Antimicrobial chlorinated 3-phenylpropanoic acid derivatives from the Red Sea marine actinomycete Streptomyces coelicolor LY001. Mar. Drugs 2020, 18, 450. [Google Scholar] [CrossRef]
- Kelly-Borges, M.; Vacelet, J. A revision of Diacarnus Burtonand and Negombata de Laubenfels (Demonspongiae: Latrunculiidae) with descriptions of new species from the west-central Pacific and Red Sea. Mem. Queensl. Mus. 1995, 38, 477–503. [Google Scholar]
- Karplus, M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Su, H.; Yuan, Z.; Li, J.; Guo, S.; Deng, L.-P.; Han, L.-J.; Zhu, X.-B.; Shi, D.Y. Sesquiterpenes from the marine red alga Laurencia saitoi. Helv. Chim. Acta 2009, 92, 1291–1297. [Google Scholar] [CrossRef]
- Yu, J.; Lin, J.; Zou, X.; Guo, H.; Li, Y. Isolation and identification of chemical constituents from marine organism Artemia cysts. Shenyang Yaoke Daxue Xuebao 2012, 29, 348–351, 358. [Google Scholar]
- Jegou, E.; Polonsky, J.; Lederer, E.; Schulte-Elte, K.H.; Egger, B.; Ohloff, G. Ambergris revisited. Isolation of volatile constituents; identification and synthesis of ambra-aldehyde C14H22O. New J. Chem. 1977, 1, 529–531. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. G09a: Gaussian 09; Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Shaala, L.A.; Youssef, D.T.A. Pseudoceratonic acid and moloka’iamine derivatives from the Red Sea Verongiid sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. [Google Scholar]
- Acar, J.F. The disc susceptibility test. In Antibiotics in Laboratory Medicine, Williams and Wilkins, Baltimore; Lorian, V., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1980; pp. 24–54. [Google Scholar]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 9th ed.; CLSI Documents M07-A9. West Valley Road, Suite 2500; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]









| No. | δC (mult.) | δH [mult., J (Hz)] | HMBC | NOESY |
|---|---|---|---|---|
| 2 | 171.5, qC | H-3 | ||
| 3 | 113.3, CH | 5.72 (s) | H3-11 | |
| 5 | 180.7, qC | H-3, H2-7, H3-11, H3-12 | ||
| 6 | 35.0, qC | H3-11, H3-12, H2-7 | ||
| 7a | 49.8, CH2 | 2.03 (ddd, 11.5, 4.2, 2.4) | H3-11, H3-12, H2-9 | |
| 7b | 1.33 (t, 11.5) | |||
| 8 | 65.1, CH | 4.13 (tt, 11.5, 4.2) | H2-7, H2-9 | H-7b, H3-12, H3-13 |
| 9a | 47.9, CH2 | 2.54 (ddd, 11.5, 4.2, 1.8) | H2-7, H3-13 | |
| 9b | 1.51 (t, 11.5) | |||
| 10 | 86.4, qC | H3-13, H-3, H2-9 | ||
| 11 | 29.9, CH3 | 1.31 (s) | H3-12 | H-3 |
| 12 | 25.1, CH3 | 1.27 (s) | H3-11 | H-8, H3-13 |
| 13 | 25.6, CH3 | 1.59 (s) | H-8, H3-12 |
| No. | δC (mult.) | δH [mult., J (Hz)] | HMBC | NOESY |
|---|---|---|---|---|
| 2 | 171.9, qC | H-3 | ||
| 3 | 112.9, CH | 5.70 (s) | H3-11 | |
| 5 | 182.3, qC | H-3, H3-11, H3-12 | ||
| 6 | 35.9, qC | H-8, H3-11, H3-12, H2-7, H-3 | ||
| 7a | 47.3, CH2 | 2.47 (td, 14.5, 3.5, 3.5) | H3-11, H3-12 | |
| 7b | 1.79 (dd, 14.5, 3.5) | H-8 | ||
| 8 | 66.8, CH | 4.33 (quin, 3.5) | H-7b, H-9a, H-9b | |
| 9a | 45.6, CH2 | 1.97 (td, 14.5, 3.5, 3.5) | H3-13 | H-8 |
| 9b | 1.53 (dd, 14.5, 3.5) | H-8 | ||
| 10 | 86.6, qC | H-3, H-8, H3-13 | ||
| 11 | 30.6, CH3 | 1.27 (s) | H3-12 | H-3 |
| 12 | 26.4, CH3 | 1.47 (s) | H3-11 | H3-13 |
| 13 | 27.0, CH3 | 1.78 (s) | H3-12 |
| No. | δC (mult.) | δH [mult., J (Hz)] | HMBC |
|---|---|---|---|
| 1 | 200.2, qC | H-6, H2-5, H3-7 | |
| 2 | 128.8, qC | H3-7, H-8 | |
| 3 | 158.6, qC | H3-7, H-9, H3-12, H3-13 | |
| 4 | 36.7, qC | H2-5, H3-12, H3-13 | |
| 5a 5b | 45.1, CH2 | 2.21 (dd, 14.0, 6.0) 1.86 (t, 14.0) | H-6, H3-12, H3-13 |
| 6 | 69.3, CH | 4.37 (dd, 14.0, 6.0) | H2-5, H3-12 |
| 7 | 13.5, CH3 | 1.88 (d, 0.6) | |
| 8 | 139.4, CH | 7.20 (dd, 16.5, 0.6) | |
| 9 | 134.1, CH | 6.22 (d, 16.5) | H-8 |
| 10 | 197.3, qC | H-8, H-9, H3-11 | |
| 11 | 28.2, CH3 | 2.36 (s) | H3-12 |
| 12 | 30.3, CH3 | 1.17 (s) | H3-13 |
| 13 | 25.7, CH3 | 1.35 (s) | H3-12, H2-5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, D.T.A.; Asfour, H.Z.; Genta-Jouve, G.; Shaala, L.A. Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a Tetrahydrooxazolo[3,2-a]azepine-2,5(3H,6H)-dione Backbone from the Red Sea Sponge Negombata magnifica. Mar. Drugs 2021, 19, 214. https://doi.org/10.3390/md19040214
Youssef DTA, Asfour HZ, Genta-Jouve G, Shaala LA. Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a Tetrahydrooxazolo[3,2-a]azepine-2,5(3H,6H)-dione Backbone from the Red Sea Sponge Negombata magnifica. Marine Drugs. 2021; 19(4):214. https://doi.org/10.3390/md19040214
Chicago/Turabian StyleYoussef, Diaa T. A., Hani Z. Asfour, Grégory Genta-Jouve, and Lamiaa A. Shaala. 2021. "Magnificines A and B, Antimicrobial Marine Alkaloids Featuring a Tetrahydrooxazolo[3,2-a]azepine-2,5(3H,6H)-dione Backbone from the Red Sea Sponge Negombata magnifica" Marine Drugs 19, no. 4: 214. https://doi.org/10.3390/md19040214