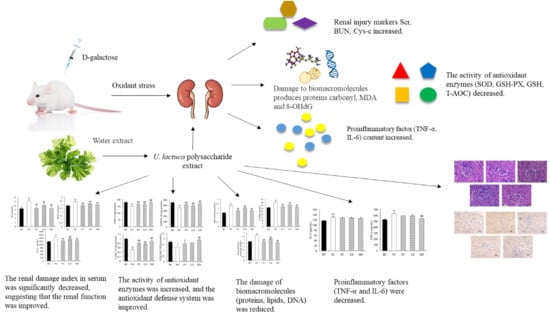
Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice
Abstract
:1. Introduction
2. Results
2.1. Characterization of UPE
2.2. Effect of UPE on Organ Index
2.3. Effects of UPE on Serum Indexes
2.4. Effects of UPE on Renal Oxidation and Antioxidant Indexes
2.5. Effects of UPE on Inflammatory Factors
2.6. Histopathological Analysis
2.7. Expression of Caspase-3 Protein in Kidney
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Characterization of UPE
4.4. Animals and Diet
4.5. Body Weight Measurement
4.6. Serum Indexes Analysis
4.7. Biochemical Analysis
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. Immunohistochemical Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chen, J.; Huang, Z.; Wu, X.; Kang, J.; Ren, Y.; Gao, W.; Lu, X.; Wang, J.; Ding, W.; Nakabeppu, Y.; et al. Oxidative stress induces different tissue dependent effects on Mutyh-deficient mice. Free Radic. Biol. Med. 2019, 143, 482–493. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [Green Version]
- Díaz, R.T.A.; Arrojo, V.C.; Agudo, M.A.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.S.E.P.W.; Pinheiro, T.S.; Castro, A.J.G.; Dore, C.M.P.G.; da Silva, N.B.; das C. Faustino Alves, M.G.; Santos, M.S.N.; Leite, E.L. Fucose-containing sulfated polysaccharides from brown macroalgae Lobophora variegata with antioxidant, anti-inflammatory, and antitumoral effects. J. Appl. Phycol. 2014, 26, 1783–1790. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Freile-Pelegrín, Y.; Robledo, D.; Moo-Puc, R. Protective effect of fucoidans from tropical seaweeds against oxidative stress in HepG2 cells. J. Appl. Phycol. 2017, 29, 2229–2238. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Jiang, N.; Liu, X.; Wan, M.; Chang, X.; Liu, D.; Qi, H. Antioxidant and antihyperlipidemic activities of purified polysaccharides from Ulva pertusa. J. Appl. Phycol. 2018, 30, 2619–2627. [Google Scholar] [CrossRef]
- Godard, M.; Décordé, K.; Ventura, E.; Soteras, G.; Baccou, J.; Cristol, J.; Rouanet, J. Polysaccharides from the green alga Ulva rigida improve the antioxidant status and prevent fatty streak lesions in the high cholesterol fed hamster, an animal model of nutritionally-induced atherosclerosis. Food Chem. 2009, 115, 176–180. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content puri fi ed polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R. Oxidant Mechanisms in Renal Injury and Disease 1,2 2 1. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Shen, Z.; Yu, J.; Yang, J.; Meng, F.; Jiang, X.; Zhu, C. Protective effects of enzyme degradation extract from Porphyra yezoensis against oxidative stress and brain injury in d -galactose-induced ageing mice. Br. J. Nutr. 2020, 123, 975–986. [Google Scholar] [CrossRef]
- El-far, A.H.; Lebda, M.A.; Noreldin, A.E.; Atta, M.S.; Elewa, Y.H.A.; Elfeky, M.; Mousa, S.A. Quercetin attenuates pancreatic and renal d- galactose-induced aging-related oxidative alterations in rats. Int. J. Mol. Sci. 2020, 21, 4348. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.X.; Xia, S.F.; Qiao, Y.; Shi, Y.H.; Le, G.W. Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet. J. Anim. Physiol. Anim. Nutr. 2015, 99, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Wang, C. Wheat bran feruloyl oligosaccharides enhance the antioxidant activity of rat plasma. Food Chem. 2010, 123, 472–476. [Google Scholar] [CrossRef]
- Jiang, G.; Lei, A.; Chen, Y.; Yu, Q.; Xie, J.; Yang, Y.; Yuan, T.; Su, D. The protective effects of the: Ganoderma atrum polysaccharide against acrylamide-induced inflammation and oxidative damage in rats. Food Funct. 2021, 12, 397–407. [Google Scholar] [CrossRef]
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative analysis of the antioxidant capacity and lipid and protein oxidation of soy and oats beverages. Food Prod. Process. Nutr. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Jnaneshwari, S.; Hemshekhar, M.; Santhosh, M.S.; Sunitha, K.; Thushara, R.; Thirunavukkarasu, C.; Kemparaju, K.; Girish, K.S. Crocin, a dietary colorant mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J. Pharm. Pharmacol. 2013, 65, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-y.; Liu, D.; Lin, G.-p.; Wu, Y.-j.; Gao, L.-y.; Ai, C.; Huang, Y.-f.; Wang, M.-f.; El-Seedi, H.R.; Chen, X.-h.; et al. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Hojs, R.; Bevc, S.; Ekart, R.; Gorenjak, M.; Puklavec, L. Serum Cystatin C as an Endogenous Marker of the Renal Function—A Review. Ren. Fail. 2008, 30, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Feng, Y.; Yu, Y.H.; Wang, S.T.; Ren, J.; Camer, D.; Hua, Y.Z.; Zhang, Q.; Huang, J.; Xue, D.L.; Zhang, X.F.; et al. Chlorogenic acid protects D-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Feng, W.; Chen, M.; Luan, H.; Hu, Y.; Zheng, X.; Wang, S.; Mao, Y. Alginate Oligosaccharide Ameliorates D-Galactose-Induced Kidney Aging in Mice through Activation of the Nrf2 Signaling Pathway. BioMed Res. Int. 2021, 2021, 6623328. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, H.; Zhang, Q. Renoprotective effect of low-molecular-weight sulfated polysaccharide from the seaweed Laminaria japonica on glycerol-induced acute kidney injury in rats. Int. J. Biol. Macromol. 2017, 95, 132–137. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Abd-Ellatef, G.E.F.; Ahmed, O.M.; Abdel-Reheim, E.S.; Abdel-Hamid, A.H.Z. Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Breast Cancer Targets Ther. 2017, 9, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.D.; Hye, J.L.; Eun, S.Y.; Shinde, P.B.; Yoon, M.L.; Hong, J.; Dong, K.K.; Jung, J.H. Anti-inflammatory constituents of the Red Alga Gracilaria verrucosa and their synthetic analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, H.R.; Zha, X.Q.; Chen, S.; Pan, L.H.; Li, Q.M.; Luo, J.P. Prevention and possible mechanism of a purified Laminaria japonica polysaccharide on adriamycin-induced acute kidney injury in mice. Int. J. Biol. Macromol. 2020, 148, 591–600. [Google Scholar] [CrossRef]
- Liu, A.D.; Zheng, K.Y.; Miao, Q.F.; Ikejima, T.; Zhang, J.; Fei, X.F. Effect of polysaccharides from fruit body of Ganoderma tsugae on bidirectional regulation of proinflammatory cytokine production in THP-1 cells. Chem. Res. Chin. Univ. 2009, 25, 487–491. [Google Scholar]
- Jiang, J.; Luo, Y.; Qin, W.; Ma, H.; Li, Q.; Zhan, J.; Zhang, Y. Electroacupuncture suppresses the NF-κB signaling pathway by upregulating cylindromatosis to alleviate inflammatory injury in cerebral ischemia/reperfusion rats. Front. Mol. Neurosci. 2017, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Kim, H.Y.; Maeng, J.; Kim, M.; Shin, D.H.; Lee, K. Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells. BMC Cancer 2014, 14, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.; Tang, L.; Yang, X.; Chen, Z.; Wei, Y.; Shao, X.; Shao, X.; Xin, Z.; Cai, B.; et al. Astragalus Polysaccharide Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Oxidative Damage and Mitochondrial Dysfunction. BioMed Res. Int. 2020, 2020, 2851349. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2019; ISBN 9781441914620. [Google Scholar]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Allen, J.; Brock, S.A. New method for quantitative determination of uronic acids. Minn. Med. 2000, 83, 45–48. [Google Scholar]
- Sorbo, B. Sulfate: Turbidimetric and Nephelometric Methods. Methods Enzymol. 1987, 143, 3–6. [Google Scholar] [CrossRef]







| Molecular Weight (kDa) | Monosaccharide Composition (%) | Composition (%) | ||
|---|---|---|---|---|
| 891.25 | Rha | 45.33% | Total sugar | 61.98 |
| GlcA | 15.50% | Protein | 2.39 | |
| Glc | 18.97% | Uronic acid | 9.13 | |
| Xyl | 20.29% | Sulfate group | 16.50 | |
| BC | NC | PC | LD | HD | |
|---|---|---|---|---|---|
| Body Weight (g) | 28.125 ± 2.064 | 27.550 ± 2.263 * | 29.300 ± 1.747 ## | 29.171 ± 4.085 ## | 27.85 ± 2.468 |
| Kidney Weight (g) | 0.306 ± 0.023 | 0.274 ± 0.019 ** | 0.307 ± 0.022 ## | 0.307 ± 0.045 ## | 0.304 ± 0.023 ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Jiang, Y.; Fu, S.; Shen, Z.; Zong, W.; Xia, Z.; Zhan, Z.; Jiang, X. Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice. Mar. Drugs 2021, 19, 539. https://doi.org/10.3390/md19100539
Yang Q, Jiang Y, Fu S, Shen Z, Zong W, Xia Z, Zhan Z, Jiang X. Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice. Marine Drugs. 2021; 19(10):539. https://doi.org/10.3390/md19100539
Chicago/Turabian StyleYang, Qian, Yanhui Jiang, Shan Fu, Zhaopeng Shen, Wenwen Zong, Zhongning Xia, Zhaoya Zhan, and Xiaolu Jiang. 2021. "Protective Effects of Ulva lactuca Polysaccharide Extract on Oxidative Stress and Kidney Injury Induced by D-Galactose in Mice" Marine Drugs 19, no. 10: 539. https://doi.org/10.3390/md19100539