The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019
Abstract
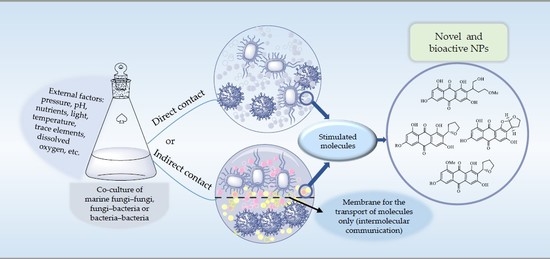
:1. Introduction
2. Compounds Derived from the Co-Cultures of Marine Microorganisms
2.1. Alkaloids
2.1.1. Alkaloids Derived from the Co-Cultures of Different Marine Fungi
2.1.2. Alkaloids Derived from the Co-Cultures of Marine Fungi and Bacteria
2.1.3. Alkaloids Derived from the Co-Cultures of Different Marine Bacteria
2.2. Anthraquinones
2.2.1. Anthraquinones Derived from the Co-Cultures of Different Marine Fungi
2.2.2. Anthraquinones Derived from the Co-Cultures of Marine Fungi and Bacteria
2.2.3. Anthraquinones Derived from the Co-Cultures of Different Marine Bacteria
2.3. Cyclopeptides
2.3.1. Cyclopeptides Derived from the Co-Cultures of Different Marine Fungi
2.3.2. Cyclopeptides Derived from the Co-Cultures of Marine Fungi and Bacteria
2.4. Macrolide
Macrolides Derived from the Co-Cultures of Different Marine Bacteria
2.5. Phenylpropanoids
2.5.1. Phenylpropanoids Derived from the Co-Cultures of Different Marine Fungi
2.5.2. Phenylpropanoids Derived from the Co-Cultures of Marine Fungi and Bacteria
2.6. Polyketides
2.6.1. Polyketides Derived from the Co-Cultures of Different Marine Fungi
2.6.2. Polyketides Derived from the Co-Cultures of Marine Fungi and Bacteria
2.6.3. Polyketides Derived from the Co-Cultures of Different Marine Bacteria
2.7. Steroids
2.7.1. Steroids Derived from the Co-Cultures of Different Marine Fungi
2.7.2. Steroids Derived from the Co-Cultures of Marine Fungi and Bacteria
2.8. Terpenoids
2.8.1. Terpenoids Derived from the Co-Cultures of Marine Fungi and Bacteria
2.8.2. Terpenoids Derived from the Co-Cultures of Different Marine Bacteria
2.9. Others
2.9.1. Other Compounds Derived from the Co-Cultures of Different Marine Fungi
2.9.2. Other Compounds Derived from the Co-Cultures of Marine Fungi and Bacteria
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Wu, Q.H.; Rowley, D.C.; Al-Kareef, A.M.; Wang, H. Anticancer agent-based marine natural products and related compounds. J. Aisan. Nat. Prod. Res. 2015, 17, 199–216. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.W.; Wang, B.X.; Lu, Y.J.; Guo, Y.Q.; Sun, J.D.; Wei, B.; Zhang, H.W.; Wang, H. Quorum sensing inhibitors from marine microorganisms and their synthetic derivatives. Mar. Drugs 2019, 17, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Okada, B.K.; Wu, Y.; Xu, F.; Seyedsayamdost, M.R. Recent advances in activating silent biosynthetic gene clusters in bacteria. Curr. Opin. Microbiol. 2018, 45, 156–163. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “one strain many compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 224. [Google Scholar] [CrossRef] [Green Version]
- Dalisay, D.S.; Rogers, E.W.; Edison, A.S.; Molinski, T.F. Structure elucidation at the nanomole scale. 1. Trisoxazole macrolides and thiazole-containing cyclic peptides from the nudibranch Hexabranchus sanguineus. J. Nat. Prod. 2009, 72, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Wolfender, J.L.; Queiroz, E.F. Chemical diversity of natural resources and the bioactivity of their constituents. CHIMIA Int. J. Chem. 2012, 66, 324–329. [Google Scholar] [CrossRef]
- Bohni, N.; Cordero-Maldonado, M.L.; Maes, J.; Siverio-Mota, D.; Marcourt, L.; Munck, S.; Kamuhabwa, A.R.; Moshi, M.J.; Esguerra, C.V.; de Witte, P.A.M.; et al. Integration of microfractionation, qNMR and zebrafish screening for the in vivo bioassay-guided isolation and quantitative bioactivity analysis of natural products. PLoS ONE 2013, 8, e64006. [Google Scholar] [CrossRef] [Green Version]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Sulieman, S.; McGaw, L. Microbial communication: A significant approach for new leads. S. Afr. J. Bot. 2017, 113, 461–470. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.S.; Majik, M.S. Bioactive alkaloids from marine sponges. In Marine Sponges: Chemicobiological and Biomedical Applications; Ehrlich, R.P.H., Ed.; Springer: New Delhi, India, 2016; pp. 257–286. [Google Scholar]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta, T.; Williams, R.M.; Tsukamoto, S. Notoamides A-D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. Engl. 2007, 46, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Samizo, M.; Nojiri, Y.; Onuki, H.; Hirota, H.; Ohta, T. Notoamides F-K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp. J. Nat. Prod. 2008, 71, 2064–2067. [Google Scholar] [CrossRef]
- Assante, G.; Camarda, L.; Locci, R.; Merlini, L.; Nasini, G.; Papadopoulos, E. Isolation and structure of red pigments from Aspergillus flavus and related species, grown on a differential medium. J. Agric. Food Chem. 1981, 29, 785–787. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.Y.; Chen, X.; Huang, M.Z.; Wan, X. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [Google Scholar] [CrossRef]
- Zhu, F.; Li, J.S.; Xie, W.C.; Shi, J.J.; Xu, F.; Song, Z.F.; Liu, Y.L. Structure revision of aspergicin by the crystal structure of aspergicine, a co-occurring isomer produced by co-culture of two mangrove epiphytic fungi. Nat. Prod. Res. 2017, 31, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Strelitz, F.; Flon, H.; Asheshovl, I.N. Antibiotic compounds with action against bacterial viruses: Neohydroxyaspergillic acid. Arch. Biochem. Biophys. 1958, 74, 150–157. [Google Scholar] [CrossRef]
- Wan, X.Q.; Zhu, F.; Chen, G.Y.; Li, H.M.; Tan, S.Y.; Pan, Y.Q.; Hong, Y. Biological evaluation of neoaspergillic acid, a pyrazine hydroxamic acid produced by mixed cultures of two marine-derived mangrove epiphytic fungi. J. Biomed. Inform. 2010, 5, 1932–1935. [Google Scholar]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.C.; Micetich, R.G.; Haskins, R.H. Antibiotic activity of neoaspergillic acid. Can. J. Microbiol. 1964, 10, 90–92. [Google Scholar] [CrossRef]
- Li, C.Y.; Ding, W.J.; Shao, C.L.; She, Z.G.; Lin, Y.C. A new diimide derivative from the co-culture broth of two mangrove fungi (strain no. E33 and K38). J. Asian Nat. Prod. Res. 2010, 12, 809–813. [Google Scholar] [CrossRef]
- Ding, W.J.; Lu, Y.C.; Feng, Z.H.; Luo, S.H.; Li, C.Y. A new nonadride derivative from the co-culture broth of two mangrove fungi. Chem. Nat. Compd. 2017, 53, 691–693. [Google Scholar] [CrossRef]
- Ebada, S.S.; Fischer, T.; Hamacher, A.; Du, F.Y.; Roth, Y.O.; Kassack, M.U.; Wang, B.G.; Roth, E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Aspergillus. Nat. Prod. Res. 2014, 28, 776–781. [Google Scholar] [CrossRef]
- Wang, J.H.; Ding, W.J.; Wang, R.M.; Du, Y.P.; Liu, H.L.; Kong, X.H.; Li, C.Y. Identification and bioactivity of compounds from the mangrove endophytic fungus Alternaria sp. Mar. Drugs 2015, 13, 4492–4504. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.U.; Asami, Y.; Lee, D.; Jang, J.-H.; Ahn, J.S.; Oh, H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. J. Nat. Prod. 2011, 74, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Asami, Y.; Jang, J.H.; Soung, N.K.; He, L.; Moon, D.O.; Kim, J.W.; Oh, H.; Muroi, M.; Osada, H.; Kim, B.Y.; et al. Protuboxepin A, a marine fungal metabolite, inducing metaphase arrest and chromosomal misalignment in tumor cells. Bioorg. Med. Chem. 2012, 20, 3799–3806. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Shi, Q.W.; Zheng, Z.H.; Ke, A.B.; Zhang, H.; Huo, C.H.; Ma, Y.; Ren, X.; Li, Y.Y.; Lin, J.; et al. Oxepinamides: Novel liver X receptor agonists from Aspergillus puniceus. Eur. J. Org. Chem. 2011, 2011, 802–807. [Google Scholar] [CrossRef]
- Yu, L.Y.; Ding, W.J.; Wang, Q.Q.; Ma, Z.J.; Xu, X.W.; Zhao, X.F.; Chen, Z. Induction of cryptic bioactive 2,5-diketopiperazines in fungus Penicillium sp. DT-F29 by microbial co-culture. Tetrahedron 2017, 73, 907–914. [Google Scholar] [CrossRef]
- Yu, L.Y.; Ding, W.J.; Ma, Z.J. Induced production of cytochalasans in co-culture of marine fungus Aspergillus flavipes and actinomycete Streptomyces sp. Nat. Prod. Res. 2016, 30, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, N.M.; Scharf, S.; Özkaya, F.C.; Kurtán, T.; Mándi, A.; Fouad, M.A.; Kamel, M.S.; Müller, W.E.G.; Kalscheuer, R.; Lin, W.H.; et al. Induction of secondary metabolites from the marine-derived fungus Aspergillus versicolor through co-cultivation with Bacillus subtilis. Planta Med. 2019, 85, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Chitsaz, F.; Abbasi, A.; Misteli, T.; Ozato, K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8758–8763. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.Y.; He, N.H.; Zhou, Q. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell Biol. 2008, 28, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, cyclotryprostatins A-D, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Terrahedron 1997, 53, 59–72. [Google Scholar] [CrossRef]
- Wang, F.Z.; Fang, Y.C.; Zhu, T.J.; Zhang, M.; Lin, A.Q.; Gu, Q.Q.; Zhu, W.M. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Thomas, E.J. Cytochalasan synthesis: Macrocycle formation via intramolecular Diels-Alder reactions. Acc. Chem. Res. 1991, 24, 229–235. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhu, T.J.; Wei, H.J.; Zhang, G.J.; Wang, H.; Gu, Q.Q. Spicochalasin A and new aspochalasins from the marine-derived fungus Spicaria elegans. Eur. J. Org. Chem. 2009, 2009, 3045–3051. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, H.Z.; Wu, N.; Liu, M.; Wei, J.T.; Zhang, Y.Y.; Lin, X.K. Characterization of the high cytochalasin E and rosellichalasin producing-Aspergillus sp. nov. F1 isolated from marine solar saltern in China. World J. Microbiol. Biotechnol. 2013, 29, 11–17. [Google Scholar] [CrossRef]
- Kalinovskaya, N.I.; Romanenko, L.A.; Irisawa, T.; Ermakova, S.P.; Kalinovsky, A.I. Marine isolate Citricoccus sp. KMM 3890 as a source of a cyclic siderophore nocardamine with antitumor activity. Microbiol. Res. 2011, 166, 654–661. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.F.; Huang, S.; Shu, Y.Z.; Vyas, D.; Fairchild, C.; Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S.E.; Gao, Q. Stephacidin A and B: Two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002, 124, 14556–14557. [Google Scholar] [CrossRef]
- Yu, L.Y.; Hu, Z.F.; Ma, Z.J. Production of bioactive tryptamine derivatives by co-culture of marine Streptomyces with Bacillus mycoides. Nat. Prod. Res. 2015, 29, 2087–2091. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Khanfar, M.A.; Rateb, M.E.; Mohammed, T.A.; Hajjar, D.; Hassan, H.M.; Gulder, T.A.M.; Abdelmohsen, U.R. New Pim-1 kinase inhibitor from the co-culture of two sponge-associated actinomycetes. Front. Chem. 2018, 6, 538. [Google Scholar] [CrossRef] [Green Version]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Mandelare, P.E.; Adpressa, D.A.; Kaweesa, E.N.; Zakharov, L.N.; Loesgen, S. Coculture of two developmental stages of a marine-derived Aspergillus alliaceus results in the production of the cytotoxic bianthrone allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.; Fredenhagen, A.; Mett, H.; Lydon, N.B.; Delmendo, R.; Jenny, H.B.; Peter, H.H. Paeciloquinones A, B, C, D, E and F: New potent inhibitors of protein tyrosine kinases produced by Paecilomyces carneus. I. Taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1995, 48, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zheng, Y.K.; Miao, C.P.; Xiong, Z.J.; Xu, L.H.; Guan, H.L.; Yang, Y.B.; Zhao, L.X. The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng. Nat. Prod. Res. 2014, 28, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.L.; Wang, X.L.; Jiang, D.F.; Wang, H.Y.; Jiao, Y.; Lou, H.X.; Wang, X.N. Metabolites from Aspergillus versicolor, an endolichenic fungus from the lichen Lobaria retigera. Drug. Discov. Ther. 2014, 8, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnani, N.; Chevrette, M.G.; Adibhatla, S.N.; Zhang, F.; Yu, Q.; Braun, D.R.; Nelson, J.; Simpkins, S.W.; McDonald, B.R.; Myers, C.L.; et al. Coculture of marine invertebrate-associated bacteria and interdisciplinary technologies enable biosynthesis and discovery of a new antibiotic, keyicin. ACS. Chem. Biol. 2017, 12, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Lin, M.Y.; Xu, D.; Lai, D.W.; Zhou, L.G. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Wang, J.H.; Luo, C.P.; Ding, W.J.; Cox, D.G. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621. [Google Scholar] [CrossRef]
- Huang, S.; Ding, W.J.; Li, C.Y.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414. [Google Scholar]
- Fremlin, L.J.; Piggott, A.M.; Lacey, E.; Capon, R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009, 72, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.; Pansy, F.E.; Perlman, D.; Smith, D.A.; Weisenborn, F.L. The in vitro activity of nonactin and its homologs: Monactin, dinactin and trinactin. J. Antibiot. 1965, 18, 128–129. [Google Scholar] [PubMed]
- Borrel, M.N.; Pereira, E.; Fiallo, M.; Garnier-Suillerot, A. Mobile ionophores are a novel class of P-glycoprotein inhibitors: The effects of ionophores on 4′-O-tetrahydropyranyl-adriamycin incorporation in K562 drug-resistant cells. Eur. J. Biochem. 1994, 223, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.J.; Strohl, W.R.; Priestley, N.D. Nonactin biosynthesis: The product of nonS catalyzes the formation of the furan ring of nonactic acid. Antimicrob. Agents Chemother. 1999, 43, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusche, B.R.; Phillips, J.B.; Priestley, N.D. Nonactin biosynthesis: Setting limits on what can be achieved with precursor-directed biosynthesis. Bioorg. Med. Chem. Lett. 2009, 19, 1233–1235. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Ma, L.; Wei, Z.P.; Han, F.; Gao, J. Advances in isolation and synthesis of xanthone derivatives. Chin. Herb. Med. 2012, 4, 87–102. [Google Scholar]
- Li, C.Y.; Zhang, J.; Shao, C.L.; Ding, W.J.; She, Z.G.; Lin, Y.C. A new xanthone derivative from the co-culture broth of two marine fungi (strain No. E33 and K38). Chem. Nat. Compd. 2011, 47, 382–384. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Wang, J.H.; Huang, S.; Li, C.Y.; Ding, W.J.; She, Z.G.; Li, C.L. A new coumarin produced by mixed fermentation of two marine fungi. Chem. Nat. Compd. 2015, 51, 239–241. [Google Scholar] [CrossRef]
- Wu, C.J.; Yi, L.; Cui, C.B.; Li, C.W.; Wang, N.; Han, X. Activation of the silent secondary metabolite production by introducing neomycin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2015, 13, 2465–2487. [Google Scholar] [CrossRef] [Green Version]
- Heguy, A.; Cai, P.; Meyn, P.; Houck, D.; Russo, S.; Michitsch, R.; Pearce, C.; Katz, B.; Bringmann, G.; Feineis, D.; et al. Isolation and characterization of the fungal metabolite 3-O-methylviridicatin as an inhibitor of tumour necrosis factor α-induced human immunodeficiency virus replication. Antivir. Chem. Chemother. 1998, 9, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Jiang, L.P.; Ge, L.; Chen, M.; Geng, C.Y.; Yang, G.; Li, Q.J.; Ji, F.; Yan, Q.; Zou, Y.; et al. Sterigmatocystin-induced oxidative DNA damage in human liver-derived cell line through lysosomal damage. Toxicol. In Vitro 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Thanislass, J.; Niranjali, S.; Devaraj, H. Lipid peroxidation as a possible secondary mechanism of sterigmatocystin toxicity. Hum. Exp. Toxicol. 2001, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Zingales, V.; Fernandez-Franzon, M.; Ruiz, M.J. The role of mitochondria in sterigmatocystin-induced apoptosis on SH-SY5Y cells. Food Chem. Toxicol. 2020, 142, 111493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, I.Y.; Park, Y.J.; Lee, J.H.; Hong, Y.S.; Lee, J.J. A novel dihydroxanthenone, AGI-B4 with inhibition of VEGF-induced endothelial cell growth. J. Antibiot. 2002, 55, 669–672. [Google Scholar] [CrossRef] [Green Version]
- Kossuga, M.H.; Ferreira, A.G.; Sette, L.D.; Berlinck, R.G.S. Two polyketides from a co-culture of two marine-derived fungal strains. Nat. Prod. Commun. 2013, 8, 721–724. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, L.P.; Zhuang, Y.B.; Kong, F.D.; Zhang, C.X.; Zhu, W.M. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29-5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef]
- Ha, T.M.; Kim, D.C.; Sohn, J.H.; Yim, J.H.; Oh, H. Anti-inflammatory and protein tyrosine phosphatase 1B inhibitory metabolites from the antarctic marine-derived fungal strain Penicillium glabrum SF-7123. Mar. Drugs. 2020, 18, 247. [Google Scholar] [CrossRef]
- Schmutz, C.; Cenk, E.; Marko, D. The alternaria mycotoxin alternariol triggers the immune response of IL-1β-stimulated, differentiated Caco-2 cells. Mol. Nutr. Food Res. 2019, 63, 1900341. [Google Scholar] [CrossRef] [Green Version]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Dellafiora, L.; Dall’Asta, C.; Cruciani, G.; Pethő, G.; Poór, M. Interaction of mycotoxin alternariol with serum albumin. Int. J. Mol. Sci. 2019, 20, 2352. [Google Scholar] [CrossRef] [Green Version]
- Anjum, K.; Sadiq, I.; Chen, L.; Kaleem, S.; Li, X.C.; Zhang, Z.Z.; Lian, X.Y. Novel antifungal janthinopolyenemycins A and B from a co-culture of marine-associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron. Lett. 2018, 59, 3490–3494. [Google Scholar] [CrossRef]
- Konoshima, T.; Takasaki, M. Anti-tumor-promoting activities (cancer chemopreventive activities) of natural products. Stud. Nat. Prod. Chem. 2000, 24, 215–267. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Organic Chemistry: Structure, Mechanism, and Synthesis; Elsevier: Boston, MA, USA, 2014. [Google Scholar]
- Roos, G.; Roos, C. Organic Chemistry Concepts: An EFL Approach; Academic Press: London, UK, 2014. [Google Scholar]
- Dias, D.A.; Urban, S. HPLC and NMR studies of phenoxazone alkaloids from Pycnoporus cinnabarinus. Nat. Prod. Commun. 2009, 4, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.E.; Raj, S.S.; Wong, S.H.; Tey, D.; Tan, H.M. Estimation of fungal growth using the ergosterol assay: A rapid tool in assessing the microbiological status of grains and feeds. Lett. Appl. Microbiol. 2008, 46, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Fischer, T.; Klassen, S.; Hamacher, A.; Roth, Y.O.; Kassack, M.U.; Roth, E.H. A new cytotoxic steroid from co-fermentation of two marine alga-derived micro-organisms. Nat. Prod. Res. 2014, 28, 1241–1245. [Google Scholar] [CrossRef]
- Wu, Q.P.; Xie, Y.Z.; Deng, Z.; Li, X.M.; Yang, W.; Jiao, C.W.; Fang, L.; Li, S.Z.; Pan, H.H.; Yee, A.J.; et al. Ergosterol peroxide isolated from Ganoderma lucidum abolishes microRNA miR-378-mediated tumor cells on chemoresistance. PLoS ONE 2012, 7, e44579. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11–Terpenoids. In Pharmacognosy Fundamentals, Applications and Strategies; Badal, S., Delgoda, R., Eds.; Academic Press: London, UK, 2017; pp. 233–266. [Google Scholar]
- Teufel, R. Unusual “head-to-torso” coupling of terpene precursors as a new strategy for the structural diversification of natural products. Methods Enzymol. 2018, 604, 425–439. [Google Scholar]
- Cho, J.Y.; Kim, M.S. Induction of antifouling diterpene production by Streptomyces cinnabarinus PK209 in co-culture with marine-derived Alteromonas sp. KNS-16. Biosci. Biotechnol. Biochem. 2012, 76, 1849–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.; Ding, W.J.; Li, C.Y.; Huang, S.P.; She, Z.G.; Lin, Y.C. New polysubstituted benzaldehyde from the co-culture broth of two marine fungi (strains Nos. E33 and K38). Chem. Nat. Compd. 2013, 49, 799–802. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Min′ko, E.M.; Ivanets, E.V.; Afiyatullov, S.S. New diorcinol J produced by co-cultivation of marine fungi Aspergillus sulphureus and Isaria felina. Chem. Nat. Compd. 2016, 52, 227–230. [Google Scholar] [CrossRef]
- Balaburski, G.M.; Leu, J.I.; Beeharry, N.; Hayik, S.; Andrake, M.D.; Zhang, G.; Herlyn, M.; Villanueva, J.; Dunbrack, R.L., Jr.; Yen, T.; et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol. Cancer Res. 2013, 11, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS. Yeast Res. 2015, 15, fov001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.X.; Wang, X.F.; Ren, G.W.; Yuan, X.L.; Deng, N.; Ji, G.X.; Li, W.; Zhang, P. Prenylated diphenyl ethers from the marine algal-derived endophytic fungus Aspergillus tennesseensis. Molecules 2018, 23, 2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.L.; Yang, H.J.; Xu, H.T.; Yin, L.Y.; Chen, Z.K.; Shen, H.H. Diphenyl ethers from a marine-derived isolate of Aspergillus sp. CUGB-F046. Nat. Prod. Res. 2018, 32, 821–825. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; Konig, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Chen, J.W.; Guo, Y.Q.; Lu, Y.J.; Wang, B.X.; Sun, J.D.; Zhang, H.W.; Wang, H. Chemistry and biology of siderophores from marine microbes. Mar. Drugs 2019, 17, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]






























| Classes | The Number of NPs | Identified Date | Bioactivities | Co-Culture of Marine Microorganisms |
|---|---|---|---|---|
| Alkaloids | 80 isolates (1–80) | 2010 and 2014–2019 | Cytotoxicity, enzyme Inhibitors, antimicrobial activities | Fungi and fungi |
| A. sulphureus KMM 4640 and I. felina KMM 4639 Aspergillus. sp. FSY-01 and FSW-02 P. citrinum SCSGAF 0052 and A. sclerotiorum SCSGAF 0053 Phomopsis sp. K38 and Alternaria sp. E33 | ||||
| Fungi and bacteria | ||||
| Penicillium sp. DT-F29 and Bacillus sp. B31 A. flavipes fungus and S. sp. CGMCC4.7185 A. fumigatus MR2012 and S. leeuwenhoekii C34 A. versicolor and B. subtilis, | ||||
| Bacteria and bacteria | ||||
| Streptomyces sp. CGMCC4.7185 and B. mycoides Saccharomonospora sp. UR22 and Dietzia sp. UR66 | ||||
| Anthraquinones | 13 isolates (81–93) | 2017–2019 | Cytotoxicity and antimicrobial activities | Fungi and fungi |
| Asexual morph and sclerotial morph of A. alliaceus | ||||
| Fungi and bacteria | ||||
| A. versicolor and B. subtilis | ||||
| Bacteria and bacteria | ||||
| Micromonospora sp. WMMB-235 and Rhodococcus sp. WMMA-185 | ||||
| Cyclopeptides | 6 isolates (94–99) | 2014 and 2019 | Antifungal and anti-proliferative activities | Fungi and fungi |
| Phomopsis sp. K38 and Alternaria sp. E33 Aspergillus sp. BM and 05-BM-05ML | ||||
| Fungi and bacteria | ||||
| A. versicolor and B. subtilis | ||||
| Macrolides | 1 isolate (100) | 2018 | Antitumor and antibacterial activity | Bacteria and bacteria |
| Saccharomonospora sp. UR22 and Dietzia sp. UR66 | ||||
| Phenylpropanoids | 23 isolates (101–123) | 2011, 2015 and 2019 | Cytotoxic, antifungal, antibacterial and anti-influenza activities | Fungi and fungi |
| Phomopsis sp. K38 and Alternaria sp. E33 A. sydowii EN-534 and P. citrinum EN-535 | ||||
| Fungi and bacteria | ||||
| A. versicolor and B. subtilis | ||||
| Polyketides | 12 isolates (124–135) | 2013, 2014 and 2018 | Anti-proliferative, cytotoxicity and antifungal activities | Fungi and fungi |
| Aspergillus sp. BM and 05 and BM-05ML Penicillium sp. Ma(M3)V and Trichoderma sp. Gc(M2)1 | ||||
| Fungi and bacteria | ||||
| Penicillium sp. WC-29-5 and S. fradiae 007 | ||||
| Bacteria and bacteria | ||||
| Janthinobacterium spp. ZZ145 and ZZ148 | ||||
| Steroids | 5 isolates (136–140) | 2009, 2010 and 2014 | Antiproliferative activity | Fungi and fungi |
| Aspergillus sp. FSY-01 and FSW-02 | ||||
| Fungi and bacteria | ||||
| Aspergillus sp. BM05 and an unknown bacteria (BM05BL) | ||||
| Terpenoids | 2 isolates (141–142) | 2012 and 2017 | Inhibition of diatom N. annexa and macroalga U. pertusa | Fungi and bacteria |
| A. fumigatus MR2012 and S. leeuwenhoekii C58 | ||||
| Bacteria and bacteria | ||||
| S. cinnabarinus PK209 and Alteromonas sp. KNS-16 | ||||
| Others | 12 isolates (143–154) | 2013, 2016, 2017 and 2019 | Antimicrobial, toxicity, cytotoxicity, Hemolytic activities | Fungi and fungi |
| Phomopsis sp. K38 and Alternaria sp. E33 P. citrinum SCSGAF 0052 and A. sclerotiorum SCSGAF 0053 A. sulphureus KMM 4640 and I. felina KMM 4639 | ||||
| Fungi and bacteria | ||||
| A. versicolor and B. subtilis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, P.; Ye, X.; Wei, B.; Emam, M.; Zhang, H.; Wang, H. The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019. Mar. Drugs 2020, 18, 449. https://doi.org/10.3390/md18090449
Chen J, Zhang P, Ye X, Wei B, Emam M, Zhang H, Wang H. The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019. Marine Drugs. 2020; 18(9):449. https://doi.org/10.3390/md18090449
Chicago/Turabian StyleChen, Jianwei, Panqiao Zhang, Xinyi Ye, Bin Wei, Mahmoud Emam, Huawei Zhang, and Hong Wang. 2020. "The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019" Marine Drugs 18, no. 9: 449. https://doi.org/10.3390/md18090449