Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia
Abstract
:1. Introduction
2. Results
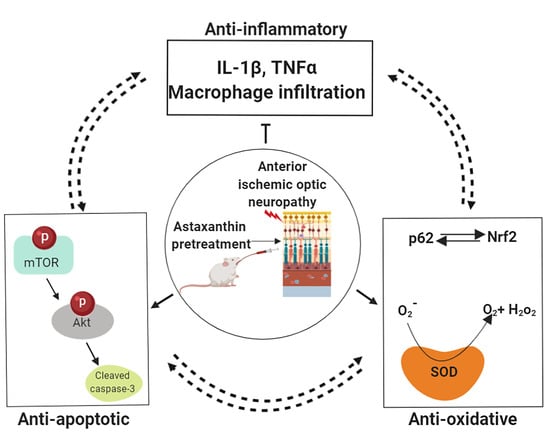
2.1. Astaxanthin Treatment Preserves Visual Function after rAION
2.2. Astaxanthin Treatment Increases RGC Survival and Reduces Apoptosis by mTOR/Akt Signaling Axis
2.3. Astaxanthin Treatment Halts Extrinsic Macrophage Infiltration in the Optic Nerve and Shows Anti-Inflammatory Potential in Retina
2.4. Astaxanthin Shows Anti-Oxidative Potential in Retina
3. Discussion
4. Methods
4.1. Animal Model and Ethics Statement
4.2. Preparation of Astaxanthin Extract from Haematococcus pluvialis
4.3. Dosage Information and Sample Size Estimation for Treatment Groups
4.4. Flash Visual-Evoked Potentials (FVEP)
4.5. Retrograde Labeling of RGCs with Fluoro-Gold
4.6. In Situ Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.7. Immunohistochemistry Staining of ED1-Positive Cells in the Optic Nerve
4.8. Western Blotting and SOD Activity Assay
4.9. Statistical Analysis and Image Editing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, A.C. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J. Neuroophthalmol. 2003, 23, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Wang, J.H.; Huang, T.L.; Tsai, R.K. Increased Risk of Stroke in Patients with Nonarteritic Anterior Ischemic Optic Neuropathy: A Nationwide Retrospective Cohort Study. Am. J. Ophthalmol. 2016, 170, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R.; Arnold, A.C. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye 2015, 29, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayreh, S.S. Pathogenesis of optic disc edema in raised intracranial pressure. Prog. Retin. Eye Res. 2016, 50, 108–144. [Google Scholar] [CrossRef] [Green Version]
- Birer, S.; Arda, H.; Kilic, D.; Baskol, G. Systemic oxidative stress in non-arteritic anterior ischemic optic neuropathy. Eye 2019, 33, 1140–1144. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Kapupara, K.; Wen, Y.T.; Tsai, R.K.; Huang, S.P. Soluble P-selectin promotes retinal ganglion cell survival through activation of Nrf2 signaling after ischemia injury. Cell Death Dis. 2017, 8, e3172. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F.; Artaria, C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S.; Wong, M.W.; Wong, T.S. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999, 19, 1849–1853. [Google Scholar] [PubMed]
- Kobayashi, M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 54, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Anuradha, C.V. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can. J. Physiol. Pharmacol. 2012, 90, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pint, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [Green Version]
- Harada, F.; Morikawa, T.; Lennikov, A.; Mukwaya, A.; Schaupper, M.; Uehara, O.; Takai, R.; Yoshida, K.; Sato, J.; Horie, Y.; et al. Protective Effects of Oral Astaxanthin Nanopowder against Ultraviolet-Induced Photokeratitis in Mice. Oxid. Med. Cell. Longev. 2017, 2017, 1956104. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, T.; Shimazawa, M.; Inoue, Y.; Nakano, Y.; Ojino, K.; Izawa, H.; Tsuruma, K.; Ishibashi, T.; Hara, H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016, 41, 1465–1472. [Google Scholar] [CrossRef]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. Protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharmacol. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Lamark, T.; Sjøttem, E.; Larsen, K.B.; Awuh, J.A.; Øvervatn, A.; McMahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, R.; Aihara, M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol. Vis. 2014, 20, 1796–1805. [Google Scholar] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur. J. Pain 2019, 23, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K. I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213. [Google Scholar] [CrossRef]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [Green Version]
- Salgado, C.; Vilson, F.; Miller, N.R.; Bernstein, S.L. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch. Ophthalmol. 2011, 129, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.T.; Huang, T.L.; Huang, S.P.; Chang, C.H.; Tsai, R.K. Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic-nerve barrier and ameliorate inflammation in a rat model of anterior ischemic optic neuropathy (rAION). Dis. Model. Mech. 2016, 9, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Slater, B.J.; Vilson, F.L.; Guo, Y.; Weinreich, D.; Hwang, S.; Bernstein, S.L. Optic nerve inflammation and demyelination in a rodent model of nonarteritic anterior ischemic optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7952–7961. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Miller, N.R.; Bernstein, S.L. Optic nerve infarction and post-ischemic inflammation in the rodent model of anterior ischemic optic neuropathy (rAION). Brain Res. 2009, 1264, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Early Methylprednisolone Treatment Can Stabilize the Blood-Optic Nerve Barrier in a Rat Model of Anterior Ischemic Optic Neuropathy (rAION). Investig. Ophthalmol. Vis. Sci. 2017, 58, 1628–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.K.; Park, Y.S.; Choi, D.K.; Chang, H.I. Effects of astaxanthin on the production of NO and the expression of COX-2 and iNOS in LPS-stimulated BV2 microglial cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [PubMed]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X.; Liu, Z. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef] [Green Version]
- Lehner, C.; Gehwolf, R.; Tempfer, H.; Krizbai, I.; Hennig, B.; Bauer, H.C.; Bauer, H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal. 2011, 15, 1305–1323. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.S.; Wang, H.D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.L.; Wang, X.L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef]
- Kapupara, K.; Huang, T.-L.; Wen, Y.-T.; Huang, S.-P.; Tsai, R.-K. Optic nerve head width and retinal nerve fiber layer changes are proper indexes for validating the successful induction of experimental anterior ischemic optic neuropathy. Exp. Eye Res. 2019, 181, 105–111. [Google Scholar] [CrossRef]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, T.L.; Huang, S.P.; Tsai, R.K. Neuroprotective effects of recombinant human granulocyte colony-stimulating factor (G-CSF) in a rat model of anterior ischemic optic neuropathy (rAION). Exp. Eye Res. 2014, 118, 109–116. [Google Scholar] [CrossRef]
- Li, X.; Qi, Z.; Zhao, L.; Yu, Z. Astaxanthin reduces type 2 diabeticassociated cognitive decline in rats via activation of PI3K/Akt and attenuation of oxidative stress. Mol. Med. Rep. 2016, 13, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Preuss, H.G.; Echard, B.; Yamashita, E.; Perricone, N.V. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: Are these effects interdependent? Int. J. Med. Sci. 2011, 8, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Meth. 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.L.; Huang, S.P.; Chang, C.H.; Lin, K.H.; Chang, S.W.; Tsai, R.K. Protective effects of systemic treatment with methylprednisolone in a rodent model of non-arteritic anterior ischemic optic neuropathy (rAION). Exp. Eye Res. 2015, 131, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.K.; Chang, C.H.; Sheu, M.M.; Huang, Z.L. Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp. Eye Res. 2010, 90, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chang, C.H.; Lin, K.H.; Sheu, M.M.; Tsai, R.K. Lack of protective effect of local administration of triamcinolone or systemic treatment with methylprednisolone against damages caused by optic nerve crush in rats. Exp. Eye Res. 2011, 92, 112–119. [Google Scholar] [CrossRef]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Efficacy of Intravitreal Injections of Triamcinolone Acetonide in a Rodent Model of Nonarteritic Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1878–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.L.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Efficacy of Intravitreal Injections of Antivascular Endothelial Growth Factor Agents in a Rat Model of Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2290–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, T.; Wen, Y.T.; Chang, C.H.; Kolovos, P.; Kalogerou, M.; Prokopiou, E.; Neokleous, A.; Huang, C. T.; Tsai, R.K. Neuroprotective Effects of Omega-3 Polyunsaturated Fatty Acids in a Rat Model of Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-N.; Kapupara, K.; Wen, Y.-T.; Chen, Y.-H.; Pan, I.-H.; Tsai, R.-K. Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Mar. Drugs 2020, 18, 85. https://doi.org/10.3390/md18020085
Lin W-N, Kapupara K, Wen Y-T, Chen Y-H, Pan I-H, Tsai R-K. Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Marine Drugs. 2020; 18(2):85. https://doi.org/10.3390/md18020085
Chicago/Turabian StyleLin, Wei-Ning, Kishan Kapupara, Yao-Tseng Wen, Yi-Hsun Chen, I-Hong Pan, and Rong-Kung Tsai. 2020. "Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia" Marine Drugs 18, no. 2: 85. https://doi.org/10.3390/md18020085