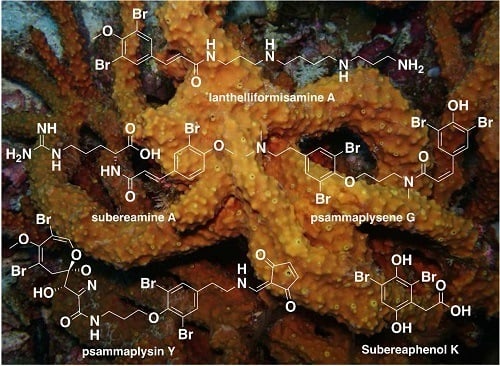
Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review
Abstract
:1. Introduction
2. Chemistry and Biological Activities of Secondary Metabolites Isolated from the Members of the genus suberea
2.1. Halogenated Tyrosine Derivatives (Isoxazolines, Oxepinisoxazolines, and Phenolics)
2.2. Non-halogenated Derivatives (Tyrosine, Aaptamine, Pyrrole, Quinolines, Isopernoids, Sesterterpenoids and Macrolides)
| Compound | Species | Local of Collection | Biological Activity | References |
|---|---|---|---|---|
| 1–5 | Suberea sp. | Okinawa | Cytotoxic, kinase inhibitors | [27] |
| 6–7 | Suberea sp. | Okinawa | Cytotoxic, antibacterial | [38] |
| 8–9 | Suberea sp. | Okinawa | Cytotoxic | [39] |
| 10–14 | Suberea sp. | Guam | Nr | [40] |
| 15–27 24 | S. aff. praetensa S. creba | Thailand Coral Sea, Australia | Cytotoxic Antiviral, antibacterial | [41,42,43] [45,46,47] |
| 28–32 32–33 23, 30–32 | S. creba S. mollis Suberea sp. | Coral Sea, Australia Red Sea, Egypt Red Sea, Egypt | Cytotoxic, antimicrobial Cytotoxic, antimicrobial Cytotoxic, antiproliferative, antibacterial | [46] [48,49] [50] |
| 34–39 | S. creba | Coral Sea, Australia | Antimicrobial, Cytotoxic, tyrosine kinase inhibitor, antiproliferative | [46,51,52,53] [54,55,56,57,58] |
| 40–41 | Suberea sp. S. mollis | Red sea, Egypt Red Sea, Egypt | Cytotoxic, antioxidant Nr | [30] [59] |
| 42–44 | S. mollis | Red Sea, Egypt | Antimicrobial | [28] |
| 45–52 | S. clavata | Great Barrier Reef, Australia | Plasma thromboplastin inhibitor | [60,61] |
| 53–57 | S. ianthelliformis | Manta Ray Bommie, Australia | Antibacterial | [29] |
| 58–62 | S. ianthelliformis | Solomon Islands | Antiplasmodial | [62] |
| 63–64 | Suberea sp. | Red Sea, Egypt | Antiproliferative | [30] |
| 65–81 | Suberea sp. | Micronesia | Cytotoxic | [63] |
| 82–91 | S. ianthelliformis | French Polynesia | Cytotoxic, acetylcholinesterase inhibitor | [34,64] |
| 92 | Suberea sp. | Lihou Reef, Australia | Cytotoxic | [65] |
| 93–94 | Suberea sp. | Red Sea, Egypt | Cytotoxic, antimicrobial | [50] |
| 95–96 | S. ianthelliformis | French Polynesia | Nr | [64] |
| 97–100 | Suberea sp. | Papua New Guinea | Human 15-Lipoxygenase inhibitor | [66] |
| 101–104 | Suberea sp. | Philippines | Antimicrobial | [67] |
| 105–107 | S. creba | New Caledonia | Cytotoxic | [68,69] |
| 108–114 | S. creba | New Caledonia | Antibacterial | [46] |
3. Proposed Biogenetic Pathways for Different Bromotyrosine Derivatives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GI50 | Half maximal growth inhibition |
| Factor XIa | Plasma thromboplastin antecedent |
| IC50 | Half maximal inhibitory concentration |
| MIC | Minimum Inhibitory concentration |
References
- Bourguet-Kondracki, M.L.; Kornprobst, J.M. Promising Marine Molecules in Pharmacology. Outstanding Marine Molecules, 1st ed.; La Barre, S., Kornprobst, J.M., Eds.; Wiley-Blackwell: Weinheim, Germany, 2014; pp. 243–264. [Google Scholar]
- Jiménez, C. Marin natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical Industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, A.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs. Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Falkenberg, M. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Bergquist, P.R. Dictyoceratida, Dendroceratida and Verongida from the New Caledonia Lagoon (Porifera: Demospongiae). Mem. Queensl. Mus. 1995, 38, 1–51. [Google Scholar]
- Hooper, J.N.A.; Van Soest, R.W.M. Systema Porifera. A Guide to the Classification of Sponges; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Gómez, P.; Bakus, G. Aplysina gerardogreeni and Aplysina aztecus (Porifera: Demospongiae) New Species from the Mexican Pacific; Anales del Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1992; pp. 175–180. [Google Scholar]
- Pulitzer-Finali, G. Some new or little-known sponges from the Great Barrier Reef of Australia. Bol. Mus. Istituti Biol. Univ. Genova 1982, 48, 87–141. [Google Scholar]
- Lendenfeld, R. Descriptive Catalogue of the Sponges in the Australian Museum, Sidney; Taylor & Francis: London, UK, 1888. [Google Scholar]
- Van Soest, R.W.M.; Kaiser, K.; van Syoc, R. Sponges from Clipperton Island, East Pacific. Zootaxa 2011, 2839, 1–46. [Google Scholar] [CrossRef]
- Hofman, C.C.; Kielman, M. The excavating sponges of the Santa-Marta area, Colombia, with description of a new species. Bijdr. Dierkd. 1992, 61, 205–217. [Google Scholar]
- Carter, H.J. Report on specimens dredged up from the Gulf of Manaar and presented to the Liverpool Free Museum by Capt. W. H. Cawne Warren. Ann. Mag. Nat. Hist. 1880, 6, 35–61. [Google Scholar] [CrossRef]
- Kelly, M.; Amirapu, S.; Mills, S.; Page, M.; Reiswig, H.M. Kermadec Islands sponge biodiversity: A review and description of a new species, Suberea meandrina sp. nov. (Demospongiae, Verongiida, Aplysinellidae). Bull. Auckland Mus. 2015, 20, 312–315. [Google Scholar]
- Row, R.W.H. Reports on the marine biology of the Sudanese Red Sea, from collections made by Cyril Crossland, M.A., B.Sc., F.Z.S. XIX. Report on the sponges collected by Mr. Cyril Crossland in 1904-5. Part II. Non-calcarea. J. Linn. Soc. Zool. 1911, 31, 287–400. [Google Scholar] [CrossRef]
- Lévi, C. Spongiaires du Vema Seamount (Atlantique Sud). Bull. Mus. Natl. Hist. Nat. 1969, 41, 952–973. [Google Scholar]
- Gugel, J.; Wagler, M.; Brümmer, F. Porifera, one new species Suberea purpureaflava n. sp. (Demospongiae, Verongida, Aplysinellidae) from northern Red Sea coral reefs, with short descriptions of Red Sea Verongida and known Suberea species. Zootaxa 2011, 2994, 60–68. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera Database. 2018. Available online: http://www.marinespecies.org/porifera (accessed on 9 January 2019).
- Hall, K.A.; Hooper, J.N.A. QM0012 Suberea ianthelliformis (Lendenfeld, 1888). SpongeMaps: An Online Community for Taxonomy and Identification of Sponges. 2014. Available online: http://www.spongemaps.org (accessed on 9 January 2019).
- Abou El-Ezz, R.; Ibrahim, A.; Habib, E.; Wahba, A.; Kamel, H.; Afifi, M.; Hassanean, H.; Ahmed, S. Review of natural products from marine organisms in the Red Sea. Int. J. Pharm. Sci. Res. 2017, 8, 940–974. [Google Scholar]
- Peng, J.; Li, J.; Hamann, M.T. The Marine Bromotyrosine Derivatives. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 61, pp. 59–262. [Google Scholar]
- Hirano, K.; Kubota, T.; Tsuda, M.; Watanabe, K.; Fromont, J.; Kobayashi, J. Ma’edamines A and B, cytotoxic bromotyrosine alkaloids with a unique 2 (1H) pyrazinone ring from Sponge Suberea sp. Tetrahedron 2002, 56, 8107–8110. [Google Scholar] [CrossRef]
- Shaala, L.A.; Bamane, F.H.; Badr, J.M.; Youssef, D.T.A. Brominated arginine-derived alkaloids from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2001, 74, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A-C, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Kherd, A. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Nicacio, K.J.; Ióca, L.P.; Fróes, A.M.; Leomil, L.; Appolinario, L.R.; Thompson, C.C.; Thompson, F.L.; Ferreira, A.G.; Williams, D.E.; Andersen, R.J.; et al. Cultures of the marine bacterium Pseudovibrio denitrificans Ab134 produce bromotyrosine-derived alkaloids previously only isolated from marine sponges. J. Nat. Prod. 2017, 80, 235–240. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Rodrigues-Stien, A.; Petek, S.; Demoy-Schnider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic guanidine alkaloids from a French Polynesian Monanchora n. sp. sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Petek, S.; Debitus, C.; Al-Mourabit, A. Unguiculins A-C: Cytotoxic bis-guanidine alkaloids from the French Polynesian sponge, Monanchora n. sp. Nat. Prod. Res. 2017, 32, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A. Isolation of Bioactive Marine Natural Products and Bio-Inspired Synthesis of Fused Guanidinic Tricyclic Analogues. Ph.D. Thesis, University of Paris-Saclay, Paris, France, 2016. [Google Scholar]
- El-Demerdash, A.; Atanasov, A.G.; Bishayee, A.; Abdel-Mogib, M.; Hooper, J.N.A.; Al-Mourabit, A. Batzella, Crambe and Monanchora: Highly prolific marine sponge genera yielding compounds with potential applications for cancer and other therapeutic areas. Nutrients 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Tammam, M.A.; Atanasov, A.G.; Hooper, J.N.A.; Al-Mourabit, A.; Kijjoa, A. Chemistry and biological activities of the marine sponges of the genera Mycale (Arenochalina), Biemna and Clathria. Mar. Drugs 2018, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Petek, S.; Debitus, C.; Al-Mourabit, A. Fatty Acids Pattern from the French Polynesian Monanchora n. sp. Marine Sponge. Chem. Nat. Compd. 2018, 54, 1134. [Google Scholar] [CrossRef]
- Tsuda, M.; Sakuma, Y.; Kobayashi, J. Suberedamines A and B, new bromotyrosine alkaloids from a sponge Suberea species. J. Nat. Prod. 2001, 64, 950–982. [Google Scholar] [CrossRef]
- Shaker, K.H.; Zinecker, H.; Ghani, M.A.; Imhoff, J.F.; Schneider, B. Bioactive metabolites from the sponge Suberea sp. Chem. Biodivers. 2010, 7, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Scupp, P.J.; Scror, J.P.; Engemann, A.; Rohde, S.; Kelmna, D.; Voogd, N.; Carroll, A.; Motti, C.A. Twilight zone sponges from Guam yield theonellin isocyanate and psammaplysins I and J. J. Nat. Prod. 2012, 75, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Kijjoa, A.; Watanadilok, R.; Sonchaeng, P.; Silva, A.M.S.; Eaton, G.; Herz, W. 11,17-Dideoxyagelorin A and B, new bromotyrosine derivatives and analogs from the marine sponge Suberea aff. praetensa. Z. Naturforsch. 2001, 56, 1116–1119. [Google Scholar] [CrossRef]
- Kijjoa, A.; Watanadilok, R.; Sonchaeng, P.; Sawangwong, P.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.S.; Eaton, G.; Herz, W. Further halotyrosine derivatives from the marine sponge Suberea aff. praetensa. Z. Naturforsch. 2002, 57, 732–738. [Google Scholar] [CrossRef]
- Kijjoa, A.; Watanadilok, R.; Sonchaeng, P.; Puchakarn, S.; Sawangwong, P.; Herz, W. Bromotyrosine derivatives from the marine sponge Suberea aff. praetensa. Bol. Mus. Ist. Biol. Univ. Genova 2004, 68, 391–397. [Google Scholar]
- Gunasekera, S.P.; Cross, S.S. Fistularin-3 and 11-ketofistularin-3. Feline leukemia virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. J. Nat. Prod. 1992, 55, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.M.; Burkholder, P.R. Studies on the antimicrobial substances of sponges II. Structure and synthesis of a bromine-containing antibacterial, compound from a marine sponge. Tetrahedron Lett. 1967, 8, 4147–4150. [Google Scholar] [CrossRef]
- Debitus, C.; Guella, G.; Mancini, I.; Waikedre, J.; Guemas, J.P.; Nicolas, J.L.; Pietra, F. Quinolones from a bacterium and tyrosine metabolites from its host sponge, Suberea creba from the Coral Sea. J. Mar. Biotechnol. 1998, 6, 136–141. [Google Scholar] [PubMed]
- Weiss, B.; Ebel, R.; Elbrächter, M.; Kirchner, M.; Proksch, P. Defense metabolites from the marine sponge Verongia aerophoba. Biochem. Syst. Ecol. 1996, 24, 1–7. [Google Scholar] [CrossRef]
- Shaala, L.A.; Khalifa, S.I.; Mesbah, M.K.; van Soest, R.W.M.; Youssef, D.T.A. Subereaphenol A, a new cytotoxic and antimicrobial dibrominated phenol from the Red Sea sponge Suberea mollis. Nat. Prod. Commun. 2008, 3, 219–222. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Gueriero, A.; Pietra, F. Novel, racemic or nearly-racemic antibacterial bromo- and chloroquinols and γ-lactams of the verongiaquinol and the cavernicolin type from the marine sponge Aplysina (=Verongia) cavernicola. HeIv. Chim. Acta 1984, 67, 1484–1492. [Google Scholar] [CrossRef]
- Shaala, L.A.; Almohammadi, A. Biologically active compounds form the Red Sea sponge Suberea sp. Pak. J. Pharm. Sci. 2017, 30, 2389–2392. [Google Scholar]
- Thomas, C.; Wolff, W.; Padmakumar, K.; Ebel, R.; Proksch, P.Z. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z. Naturforsch. 2004, 59c, 113–122. [Google Scholar] [CrossRef]
- Fattorusso, E.; Minale, L.; Sodano, G. Aeroplysinin-1, an antibacterial bromo-compound from the sponge Verongia aerophoba. Chem. Soc. Perkin Trans. 1972, 1, 16–18. [Google Scholar] [CrossRef]
- Fulmor, W.; Van Lear, G.E.; Morton, G.O.; Mills, R.D. Isolation and absolute configuration of the aeroplysinin I enantiomorphic pair from Ianthella ardis. Tetrahedron Lett. 1970, 11, 4551–4552. [Google Scholar] [CrossRef]
- Teeyapant, R.; Woerdenbag, H.J.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch. 1993, 48, 939–945. [Google Scholar] [CrossRef]
- Koulman, A.; Proksch, P.; Ebel, R.; Beekman, A.C.; van Uden, W.; Konings, A.W.; Pedersen, J.A.; Pras, N.; Woerdenbag, H.J. Cytoxicity and mode of action of aeroplysinin-1 and a related dienone from the sponge Aplysina aerophoba. J. Nat. Prod. 1996, 59, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.H.; Leake, R.E.; Rinaldi, F.; Müller-Klieser, W.; Maidhof, A.; Müller, W.E.G.; Schröder, H.C. Inhibition of intrinsic protein tyrosine kinase activity of EGF-receptor kinase complex from human breast cancer cells by the marine sponge metabolite (+)-aeroplysinin-1. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1990, 97, 151–158. [Google Scholar] [CrossRef]
- Martinez-Poveda, B.; Garcia-Vilas, J.A.; Cardenas, C.; Melgarejo, E.; Quesada, A.R.; Medina, M.A. The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro-inflammatory molecules in human endothelial and monocyte cells. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hinterdinga, K.; Knebelb, A.; Herrlichb, P.; Waldmanna, H. Synthesis and biological evaluation of aeroplysinin analogues: A new class of receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. 1998, 6, 1153–1162. [Google Scholar] [CrossRef]
- Abou-Ashour, M.I.; Shaala, L.A.; Youssef, D.T.A.; Bader, J.M.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Xue, Y.; Oster, L.; Fex, T.; et al. Clavatadine A, a natural product with selective recognition and irreversible inhibition of factor Xia. J. Med. Chem. 2008, 51, 3583–3587. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Hooper, J.N.A.; Quinn, R.J. Clavatadines C-E, guanidine alkaloids from the Australian sponge Suberea clavata. J. Nat. Prod. 2009, 72, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.A.; Erpenbeck, D.; Aalbersberg, W.; et al. New antiplasmodial bromotyrosine derivatives from Suberea ianthelliformis (Lendenfeld, 1888). Chem. Biodivers. 2012, 9, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Han, S.; Lee, H.S.; Kang, J.S.; Yun, J.; Sim, C.J.; Shin, H.J.; Le, J.S. Cytotoxic psammaplysin analogues from a Suberea sp. marine sponge and the role of the spirooxepinisoxazoline in their activity. J. Nat. Prod. 2013, 76, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Moriou, C.; Toullec, J.; Besson, M.; Soulet, S.; Schmitt, N.; Petek, S.; Lecchini, D.; Debitus, C.; Al-Mourabit, A. Bioactive bromotyrosine-derived alkaloids from the Polynesian sponge Suberea ianthelliformis. Mar. Drugs 2018, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; McCool, B.J.; Willis, R.H. Lihouidine, a novel spiro polycyclic aromatic alkaloids from the marine sponge Suberea n. sp. (Aplysinellidae, Verongida). J. Org. Chem. 2004, 69, 7791–7793. [Google Scholar] [CrossRef]
- Carroll, J.; Jonsson, E.N.; Ebel, R.; Hartman, M.S.; Holman, T.R.; Crews, P. Probing sponge-derived terpenoids for human 15-lipoxgenase inhibitors. J. Org. Chem. 2001, 66, 6847–6851. [Google Scholar] [CrossRef]
- Lee, J.; Shin, A.Y.; Lee, H.S. Isolation and synthesis of luffariellolide derivatives and evaluation of antibacterial activities against Gram-Negative bacteria. Bull. Korean Chem. Soc. 2017, 38, 804–807. [Google Scholar] [CrossRef]
- Carletti, I.; Massiot, G. Macrolides Useful as Anticancer Agents. U.S. Patent WO2014114729 A1, 31 December 2015. Available online: https://patents.google.com/patent/US20150376222A1/en (accessed on 9 January 2019).
- Carletti, I.; Massiot, G. Macrolides Useful as Anticancer Agents. U.S. Patent US9873715B2, 23 January 2018. Available online: https://patents.google.com/patent/US9873715B2/en (accessed on 9 January 2019).
- Shimizu, Y. Paralytic shellfish poisons. Prog. Chem. Org. Nat. Prod. 1984, 45, 235. [Google Scholar]
- Butler, A.; Walker, J.V. Marine haloperoxidases. Chem. Rev. 1993, 93, 1937. [Google Scholar] [CrossRef]
- Mani, L. The isolation and characterization of antibacterial compounds from the marine sponge, Suberea clavata. Master’s Thesis, University of the South Pacific, Suva, Fiji, 14 June 2005. [Google Scholar]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs 2019, 17, 115. https://doi.org/10.3390/md17020115
El-Demerdash A, Atanasov AG, Horbanczuk OK, Tammam MA, Abdel-Mogib M, Hooper JNA, Sekeroglu N, Al-Mourabit A, Kijjoa A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Marine Drugs. 2019; 17(2):115. https://doi.org/10.3390/md17020115
Chicago/Turabian StyleEl-Demerdash, Amr, Atanas G. Atanasov, Olaf K. Horbanczuk, Mohamed A. Tammam, Mamdouh Abdel-Mogib, John N. A. Hooper, Nazim Sekeroglu, Ali Al-Mourabit, and Anake Kijjoa. 2019. "Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review" Marine Drugs 17, no. 2: 115. https://doi.org/10.3390/md17020115