Increased Cell Detachment Ratio of Mesenchymal-Type Lung Cancer Cells on pH-Responsive Chitosan through the β3 Integrin
Abstract
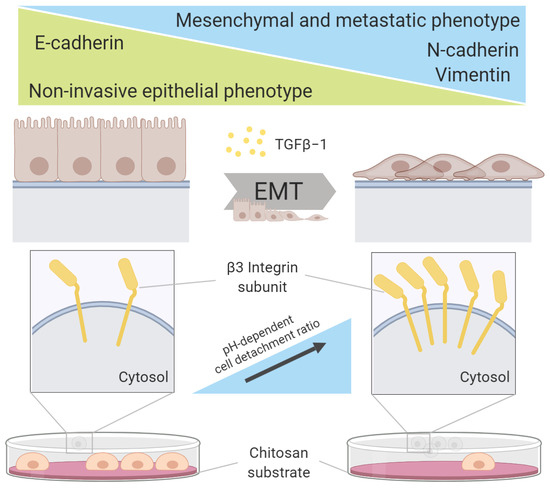
:1. Introduction
2. Results
2.1. The Effect of Transforming Growth Factor-β1 (TGF-β1) on Cellular Morphology
2.2. Immunocytochemistry and Western Blots Analyses of EMT
2.3. CDR of A549 Cells on pH-Responsive Chitosan
2.4. Expressions of Integrin in EMT-Induced A549 Cells and the Effect of Integrin Inhibitors on CDR
2.5. CDR of Heterogeneous Cell Mixture on pH-Responsive Chitosan
3. Discussion
4. Materials and Methods
4.1. Preparation of Chitosan Substrate
4.2. Medium Preparation and Cell Culture
4.3. Morphometric Measurement
4.4. Immunocytochemical Staining
4.5. Immunoblotting
4.6. Cell Detachment Assay and Cell Detachment Ratio
4.7. Detection of Cell Apoptosis
4.8. Integrin Protein Array
4.9. Integrin-Mediated Functional Inhibition
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Longley, R.L.; Woods, A.; Fleetwood, A.; Cowling, G.J.; Gallagher, J.T.; Couchman, J.R. Control of morphology, cytoskeleton and migration by syndecan-4. J. Cell Sci. 1999, 112, 3421–3431. [Google Scholar]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Transduction—Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ’seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Fidler, I.J. Critical determinants of metastasis. Semin. Cancer Biol. 2002, 12, 89–96. [Google Scholar] [CrossRef]
- Liotta, L.; Lee, C.; Morakis, D. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980, 11, 141–152. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.; Grandmaison, E.; Goosen, M. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Lin, J.C. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection–deprotection strategy. J. Biomater. Sci. Polym. Ed. 2008, 19, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chung, Y.C.; Wang, I.J.; Young, T.H. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials 2012, 33, 1336–1342. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, S.H.; Wang, T.J.; Wang, I.J.; Young, T.H. Cell fractionation on pH-responsive chitosan surface. Biomaterials 2013, 34, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.G.; Kojima, S.I.; Goldman, R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: A versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, S.M.; Kang, J.H.; Chung, B.G.; Lee, J.M.; Chung, I.Y. Epithelial-to-mesenchymal transition of human lung alveolar epithelial cells in a microfluidic gradient device. Electrophoresis 2013, 34, 441–447. [Google Scholar] [CrossRef]
- Tirino, V.; Camerlingo, R.; Bifulco, K.; Irollo, E.; Montella, R.; Paino, F.; Sessa, G.; Carriero, M.V.; Normanno, N.; Rocco, G.; et al. TGF-beta 1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction. Cell Death Dis. 2013, 4, e620. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.C.; Hsu, S.F.; Wu, C.L.; Li, T.M.; Kao, S.T.; Tsai, F.J.; Chen, W.C.; Liu, S.C.; Wu, C.M.; Tang, C.H. Transforming growth factor-beta 1 increases cell migration and beta 1 integrin up-regulation in human lung cancer cells. Lung Cancer 2009, 64, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.X.; Fang, J.; Tong, S.J.; Qu, L.X.; Jiang, H.W.; Ding, Q.; Liu, J. Over-Expression of Lipocalin 2 Promotes Cell Migration and Invasion Through Activating ERK Signaling to Increase SLUG Expression in Prostate Cancer. Prostate 2015, 75, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Suppression of DACH1 promotes migration and invasion of colorectal cancer via activating TGF-beta-mediated epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2015, 460, 314–319. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Tooney, N.M. Solution and surface effects on plasma fibronectin structure. J. Cell Biol. 1983, 97, 1686–1692. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Tarone, G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003, 19, 173–206. [Google Scholar] [CrossRef]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Gong, J.; Wang, D.; Sun, L.; Zborowska, E.; Willson, J.K.; Brattain, M.G. Role of alpha 5 beta 1 integrin in determining malignant properties of colon carcinoma cells. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 1997, 8, 83–90. [Google Scholar]
- Hofmann, U.B.; Westphal, J.R.; Waas, E.T.; Becker, J.C.; Ruiter, D.J.; van Muijen, G.N.P. Coexpression of integrin αvβ3 and matrix metalloproteinase-2 (MMP-2) coincides with MMP-2 activation: Correlation with melanoma progression. J. Investig. Dermatol. 2000, 115, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Sievers, E.; Hou, Y.P.; Park, R.; Tohme, M.; Bart, R.; Bremner, R.; Bading, J.R.; Conti, P.S. Integrin αvβ3-targeted imaging of lung cancer. Neoplasia 2005, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gladson, C.L.; Cheresh, D.A. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J. Clin. Investig. 1991, 88, 1924–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolli, M.; Fransvea, E.; Pilch, J.; Saven, A.; Felding-Habermann, B. Activated integrin alpha v beta 3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9482–9487. [Google Scholar] [CrossRef] [Green Version]
- Seftor, R.E.; Seftor, E.A.; Gehlsen, K.R.; Stetler-Stevenson, W.G.; Brown, P.D.; Ruoslahti, E.; Hendrix, M.J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc. Natl. Acad. Sci. USA 1992, 89, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Khatau, S.B.; Hale, C.M.; Stewart-Hutchinson, P.J.; Patel, M.S.; Stewart, C.L.; Searson, P.C.; Hodzic, D.; Wirtz, D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA 2009, 106, 19017–19022. [Google Scholar] [CrossRef] [Green Version]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-H.; Young, T.-H.; Hsieh, M.-C.; Liao, L.-J.; Huang, T.-W. Increased Cell Detachment Ratio of Mesenchymal-Type Lung Cancer Cells on pH-Responsive Chitosan through the β3 Integrin. Mar. Drugs 2019, 17, 659. https://doi.org/10.3390/md17120659
Yen C-H, Young T-H, Hsieh M-C, Liao L-J, Huang T-W. Increased Cell Detachment Ratio of Mesenchymal-Type Lung Cancer Cells on pH-Responsive Chitosan through the β3 Integrin. Marine Drugs. 2019; 17(12):659. https://doi.org/10.3390/md17120659
Chicago/Turabian StyleYen, Chia-Hsiang, Tai-Horng Young, Meng-Chi Hsieh, Li-Jen Liao, and Tsung-Wei Huang. 2019. "Increased Cell Detachment Ratio of Mesenchymal-Type Lung Cancer Cells on pH-Responsive Chitosan through the β3 Integrin" Marine Drugs 17, no. 12: 659. https://doi.org/10.3390/md17120659