Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and Their Sponge-Prey Acanthella cavernosa
Abstract
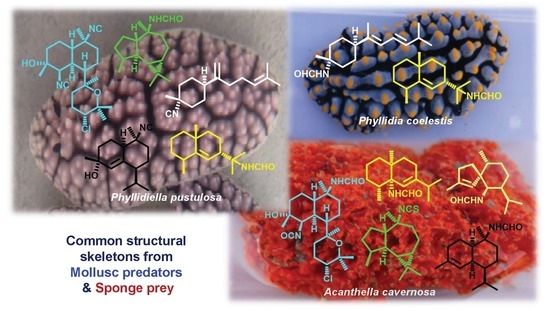
:1. Introduction
2. Results
2.1. Phyllidiella pustulosa
2.2. Phyllidia coelestis
2.3. Acanthella cavernosa
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material, Extraction, and Isolation
4.2.1. Biological Material
4.2.2. Extraction and Isolation of 1–19
4.3. Bioassay Procedures
Cytotoxic Activity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzo, E.; Ciavatta, M.L.; Gavagnin, M.; Mollo, E.; Guo, Y.-W.; Cimino, G. Isocyanide terpene metabolites of Phyllidiella pustulosa, a nudibranch from the South China Sea. J. Nat. Prod. 2004, 67, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Jomoria, T.; Shibutani, T.; Ahmadi, P.; Suzuka, T.; Tanaka, J. A New Isocyanosesquiterpene from the nudibranch Phyllidiella pustulosa. Nat. Prod. Commun. 2015, 10, 1913–1914. [Google Scholar] [PubMed]
- Jaisamut, S.; Prabpai, S.; Tancharoen, C.; Yuenyongsawad, S.; Hannongbua, S.; Kongsaeree, P.; Plubrukarn, A. Bridged tricyclic sesquiterpenes from the tubercle nudibranch Phyllidia coelestis Bergh. J. Nat. Prod. 2013, 76, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Pierens, G.K.; Skinner-Adams, T.; Andrews, K.T.; Bernhardt, P.V.; Krenske, E.H.; Mollo, E.; Garson, M.J. Antimalarial isocyano and isothiocyanato sesquiterpenes with tri- and bicyclic skeletons from the nudibranch Phyllidia ocellata. J. Nat. Prod. 2015, 78, 1422–1427. [Google Scholar] [CrossRef]
- Sim, D.C.; Wayan, M.I.; White, A.M.; Martiningsih, N.W.; Loh, J.J.M.; Cheney, K.L.; Garson, M.J. New sesquiterpenoid isonitriles from three species of Phyllidid nudibranchs. Fitoterapia 2017, 126, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yasman, Y.; Edrada, R.A.; Wray, V.; Proksch, P. New 9-thiocyanatopupukeanane sesquiterpenes from the nudibranch Phyllidia varicosa and its sponge-prey Axinyssa aculeata. J. Nat. Prod. 2003, 66, 1512–1514. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Flowers, A.E.; Garson, M.J.; Moore, C.J. The biosynthesis of sesquiterpene isocyanides and isothiocyanates in the marine sponge Acanthella cavernosa (Dendy); Evidence for dietary transfer to the dorid nudibranch Phyllidiella pustulosa. Comp. Biochem. Phys. 1997, 118, 1385–1392. [Google Scholar] [CrossRef]
- Thompson, J.E.; Walker, R.P.; Wratten, S.J.; Faulkner, D.J. A chemical defense mechanism for the nudibranch Cadlina luteomarginata. Tetrahedron 1982, 38, 1865–1873. [Google Scholar] [CrossRef]
- Gulavita, N.K.; de Silva, E.D.; Hagadone, M.R.; Karuso, P.; Scheuer, P.J. Nitrogenous bisabolene sesquiterpenes from marine invertebrates. J. Org. Chem. 1986, 51, 5136–5139. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Matsunaga, S. Two new sesquiterpene isonitriles from the nudibranch, Phyllida pustulosa. Tetrahedron Lett. 1991, 32, 7291–7294. [Google Scholar] [CrossRef]
- Kassuhlke, K.E.; Potts, B.C.M.; Faulkner, D.J. New nitrogenous sesquiterpenes from two Philippine nudibranchs, Phyllidia pustulosa and P. varicosa, and from a Palauan sponge, Halichondria cf. lendenfeldi. J. Org. Chem. 1991, 56, 3747–3750. [Google Scholar] [CrossRef]
- Hirota, H.; Okino, T.; Yoshimura, E.; Fusetani, N. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron 1998, 54, 13971–13980. [Google Scholar] [CrossRef]
- Wright, A.D. GC-MS and NMR analysis of Phyllidiella pustulosa and one of its dietary sources, the sponge Phakellia carduus. Comp. Biochem. Physiol. 2003, 134, 307–313. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Qi, S.-H.; Song, S.-J.; Chen, W.-S.; Lin, H.-W. Antifouling and cytotoxic constituents from the South China Sea sponge Acanthella cavernosa. Tetrahedron 2012, 68, 2876–2883. [Google Scholar] [CrossRef]
- Xu, Y.; Lang, J.-H.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Song, S.-J.; Zhang, B.-H.; Lin, H.-W. Formamido-diterpenes from the South China Sea sponge Acanthella cavernosa. Mar. Drugs 2012, 10, 1445–1458. [Google Scholar] [CrossRef]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Z.; Chen, K.-S.; Yao, L.-G.; Liu, H.-L.; Guo, Y.-W. A new kalihinol diterpene from the Hainan sponge Acanthella sp. Arch. Pharm. Res. 2009, 32, 1581–1584. [Google Scholar] [CrossRef]
- Yan, X.-H.; Zhu, X.-Z.; Yu, J.-L.; Jin, D.-Z.; Guo, Y.-W.; Mollo, E.; Cimino, G. 3-Oxo-axisonitrile-3, a new sesquiterpene isocyanide from the Chinese marine sponge Acanthella sp. J. Asian Nat. Prod. Res. 2006, 8, 579–584. [Google Scholar] [CrossRef]
- Miyaoka, H.; Shimomura, M.; Kimura, H.; Yamada, Y. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge Acanthella sp. Tetrahedron 1998, 54, 13467–13474. [Google Scholar] [CrossRef]
- Angerhofer, C.K.; Pezzuto, J.M.; König, G.M.; Wright, A.D.; Sticher, O. Antimalarial activity of sesquiterpenes from the marine sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 1787–1789. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Faulkner, D.J. Six new diterpene isonitriles from the sponge Acanthella cavernosa. J. Nat. Prod. 1994, 57, 501–506. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K. New isothiocyanate sesquiterpenes from the Australian marine sponge Acanthella pulcherrima. Aust. J. Chem. 1988, 41, 979–983. [Google Scholar] [CrossRef]
- Yang, L.-H.; Lee, O.O.; Jin, T.; Li, X.-C.; Qian, P.-Y. Antifouling properties of 10beta-formamidokalihinol-A and kalihinol A isolated from the marine sponge Acanthella cavernosa. Biofouling 2006, 22, 23–32. [Google Scholar] [CrossRef]
- Nogata, Y.; Yoshimura, E.; Shinshima, K.; Kitano, Y.; Sakaguchi, I. Antifouling substances against larvae of the barnacle Balanus amphitrite from the marine sponge, Acanthella cavernosa. Biofouling 2003, 19, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling kalihipyrans from the marine sponge Acanthella cavernosa. J. Nat. Prod. 1996, 59, 1081–1083. [Google Scholar] [CrossRef]
- Fusetani, N.; Hiroto, H.; Okino, T.; Tomono, Y.; Yoshimura, E. Antifouling activity of isocyanoterpenoids and related compounds isolated from a marine sponge and nudibranchs. J. Nat. Toxins 1996, 5, 249–259. [Google Scholar]
- Hirota, H.; Tomono, Y.; Fusetani, N. Terpenoids with antifouling activity against barnacle larvae from the marine sponge Acanthella cavernosa. Tetrahedron 1996, 52, 2359–2368. [Google Scholar] [CrossRef]
- Nishikawa, K.; Umezawa, T.; Garson, M.J.; Matsuda, F. Confirmation of the configuration of 10-isothiocyanato-4-cadinene diastereomers through synthesis. J. Nat. Prod. 2012, 75, 2232–2235. [Google Scholar] [CrossRef]
- Miyaoka, H.; Abe, Y.; Sekiya, N.; Mitome, H.; Kawashima, E. Total synthesis of antimalarial diterpenoid (+)-kalihinol A. Chem. Commun. 2012, 48, 901–903. [Google Scholar] [CrossRef]
- White, R.D.; Keaney, G.F.; Slown, C.D.; Wood, J.L. Total synthesis of (+/-)-kalihinol C. Org. Lett. 2004, 6, 1123–1126. [Google Scholar] [CrossRef]
- White, R.D.; Wood, J.L. Progress toward the total synthesis of kalihinane diterpenoids. Org. Lett. 2001, 3, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Chen, S.-H.; Ye, F.; Mollo, E.; Zhu, W.-L.; Liu, H.-L.; Guo, Y.-W. Axiriabilines A–D, uncommon nitrogenous eudesmane-type sesquiterpenes from the Hainan sponge Axinyssa variabilis. Tetrahedron 2017, 73, 5239–5243. [Google Scholar] [CrossRef]
- Liu, H.-L.; Xue, D.-Q.; Chen, S.-H.; Li, X.-W.; Guo, Y.-W. New highly oxidized formamidobisabolene-derived sesquiterpenes from a Hainan sponge Axinyssa variabilis. Helv. Chim. Acta 2016, 99, 650–653. [Google Scholar] [CrossRef]
- Wu, Q.; Nay, B.; Yang, M.; Ni, Y.; Wang, H.; Yao, L.; Li, X. Marine sponges of the genus Stelletta as promising drug sources: Chemical and biological aspects. Acta Pharm. Sin. B 2018. [Google Scholar] [CrossRef]
- Prawat, H.; Mahidol, C.; Kaweetripob, W.; Prachyawarakorn, V.; Tuntiwachwuttikul, P.; Ruchirawat, S. Sesquiterpene isocyanides, isothiocyanates, thiocyanates, and formamides from the Thai sponge Halichondria sp. Tetrahedron 2016, 72, 4222–4229. [Google Scholar] [CrossRef]
- Clark, R.J.; Stapleton, B.L.; Garson, M.J. New isocyano and isothiocyanato terpene metabolites from the tropical marine sponge Acanthella cavernosa. Tetrahedron 2000, 56, 3071–3076. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakahara, H.; Shirokura, Y.; Nogata, Y.; Yoshimura, E.; Umezawa, T.; Okino, T.; Matsuda, F. Total synthesis of 10-isocyano-4-cadinene and determination of its absolute configuration. Org. Lett. 2010, 12, 904–907. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakahara, H.; Shirokura, Y.; Nogata, Y.; Yoshimura, E.; Umezawa, T.; Okino, T.; Matsuda, F. Total synthesis of 10-isocyano-4-cadinene and its stereoisomers and evaluations of antifouling activities. J. Org. Chem. 2011, 76, 6558–6573. [Google Scholar] [CrossRef]
- Petrichtcheva, N.V.; Duque, C.; Dueñas, A.; Zea, S.; Hara, N.; Fujimoto, Y. New nitrogenous eudesmane-type compounds isolated from the Caribbean sponge Axinyssa ambrosia. J. Nat. Prod. 2002, 65, 851–855. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mayol, L. Nitrogenous sesquiterpenes based on allo-aromadendrane and epi-eudesmane skeletons from the marine sponge Axinella cannabina. Can. J. Chem. 1987, 65, 518–522. [Google Scholar] [CrossRef]
- Zubía, E.; Ortega, M.J.; Carballo, J.L. Sesquiterpenes from the sponge Axinyssa isabela. J. Nat. Prod. 2008, 71, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Jumaryatno, P.; Stapleton, B.L.; Hooper, J.N.A.; Brecknell, D.J.; Blanchfield, J.T.; Garson, M.J. A comparison of sesquiterpene scaffolds across different populations of the tropical marine sponge Acanthella cavernosa. J. Nat. Prod. 2007, 70, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Prawat, H.; Mahidol, C.; Wittayalai, S.; Intachote, P.; Kanchanapoom, T.; Ruchirawat, S. Nitrogenous sesquiterpenes from the Thai marine sponge Halichondria sp. Tetrahedron 2011, 67, 5651–5655. [Google Scholar] [CrossRef]
- Zhang, W.; Gavagnin, M.; Guo, Y.-W.; Mollo, E.; Ghiselinc, M.T.; Cimino, G. Terpenoid metabolites of the nudibranch Hexabranchus sanguineus from the South China Sea. Tetrahedron 2007, 63, 4725–4729. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling kalihinenes from the marine sponge Acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640. [Google Scholar] [CrossRef]
- Zubía, E.; Ortega, M.J.; Hernández-Guerrero, C.J.; Carballo, J.L. Isothiocyanate sesquiterpenes from a sponge of the genus Axinyssa. J. Nat. Prod. 2008, 71, 608–614. [Google Scholar] [CrossRef]
- Rodriguez, J.; Nieto, R.M.; Hunter, L.M.; Diaz, M.C.; Crews, P.; Lobkovsky, E.; Clardy, J. Variation among known kalihinol and new kalihinene diterpenes from the sponge Acanthella cavernosa. Tetrahedron 1994, 50, 11079–11090. [Google Scholar] [CrossRef]
- Shimomura, M.; Miyaoka, H.; Yamada, Y. Absolute configuration of marine diterpenoid kalihinol A. Tetrahedron Lett. 1999, 40, 8015–8017. [Google Scholar] [CrossRef]
- He, W.-F.; Li, Y.; Feng, M.-T.; Gavagnin, M.; Mollo, E.; Mao, S.C.; Guo, Y.-W. New isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its possible sponge-prey Xestospongia sp. Fitoterapia 2014, 96, 109–114. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Chen, W.-T.; Kurtán, T.; Mándi, A.; Ding, J.; Li, J.; Li, X.-W.; Guo, Y.-W. Bioactive isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its sponge-prey Xestospongia sp. Future Med. Chem. 2016, 8, 17–27. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Manzo, E.; Mollo, E.; Mattia, C.A.; Tedesco, C.; Irace, C.; Guo, Y.-W.; Li, X.-B.; Cimino, G.; Gavagnin, M. Tritoniopsins A–D, cladiellane-based diterpenes from the South China Sea nudibranch Tritoniopsis elegans and its prey Cladiella krempfi. J. Nat. Prod. 2011, 74, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-C.; Guo, Y.-W.; van Soest, R.; Cimino, G. New nitrogenous bisabolene-type sesquiterpenes from a Hainan sponge Axinyss aff. Variabilis. Helv. Chim. Acta 2007, 90, 588–593. [Google Scholar] [CrossRef]




| No. | 1 | 20 | No. | 16 | 17 | ||
|---|---|---|---|---|---|---|---|
| δH Mult (J in Hz) | δC | δC | δH Mult (J in Hz) | δC | δC | ||
| 1 | 1.96 m | 45.9 CH | 46.9 CH | 1 | 1.37 m | 42.6 CH | 42.3 CH |
| 2a | 1.69 m | 21.6 CH2 | 21.6 CH2 | 2a | 1.47 m | 21.7 CH2 | 21.6 CH2 |
| 2b | 1.94 m | 2b | 1.61 m | ||||
| 3a | 1.53 m | 36.3 CH2 | 36.2 CH2 | 3 | 1.51 m, 2H | 33.7 CH2 | 32.6 CH2 |
| 3b | 1.96 m | ||||||
| 4 | - | 69.5 qC | 69.3 qC | 4 | - | 71.6 qC | 70.5 qC |
| 5 | 5.58 s | 130.4 CH | 130.2 CH | 5 | 4.18 (d, 10.4) | 59.8 CH | 63.7 CH |
| 6 | - | 136.5 qC | 137.2 qC | 6 | 2.35 m | 36.6 CH | 36.0 CH |
| 7 | 1.64 m | 47.3 CH | 47.4 CH | 7 | 1.57 m | 45.8 CH | 48.4 CH |
| 8a | 1.49 m | 21.8 CH2 | 22.5 CH2 | 8a | 1.62 m | 23.1 CH2 | 21.9 CH2 |
| 8b | 1.69 m | 8b | 1.02 m | ||||
| 9a | 1.51 m | 39.4 CH2 | 40.4 CH2 | 9a | 1.72 m | 40.7 CH2 | 39.7 CH2 |
| 9b | 2.01 (d, 10.0) | 9b | 1.55 m | ||||
| 10 | - | 63.3 qC | 66.0 qC | 10 | - | 55.0 qC | 59.0 qC |
| 11 | 2.14 m | 26.8 CH | 26.8 CH | 11 | - | 79.0 qC | 76.8 qC |
| 12 | 0.97 (d, 6.8) | 22.1 CH3 | 22.1 CH | 12a | 1.48 m | 38.1 CH2 | 38.0 CH2 |
| 12b | 1.57 m | ||||||
| 13 | 0.90 (d, 6.8) | 17.5 CH3 | 17.5 CH | 13a | 1.99 m | 27.7 CH2 | 27.4 CH2 |
| 13b | 2.06 m | ||||||
| 14 | 1.40 s | 27.1 CH3 | 26.7 CH3 | 14 | 3.68 (dd, 12.4, 4.4) | 64.4 CH | 64.1 CH |
| 15 | 1.42 (t, 1.8) | 28.9 CH3 | 28.2 CH3 | 15 | - | 76.7 qC | 76.0 qC |
| NC (1) and NCS (20) | - | n.d. b | n.d. b | 16 | 1.37 s | 23.5 CH3 | 22.8 CH3 |
| 17 | 1.31 s | 31.4 CH3 | 30.5 CH3 | ||||
| 18 | 1.27 s | 19.7 CH3 | 19.2 CH3 | ||||
| 19 | 1.19 s | 18.8 CH3 | 29.0 CH3 | ||||
| 20 | 1.18 s | 29.0 CH3 | 20.7 CH3 | ||||
| CHO-1 or NC | 8.25 (d, 12.0) | 163.7 CH | 157.0 qC | ||||
| CHO-2 or NC | 8.10 (d, 11.4) | 167.6 CH | 153.0 qC | ||||
| Compounds a | A549 | HT-29 | SNU-398 | Capan-1 |
|---|---|---|---|---|
| IC50 (μM) | ||||
| 8 | 8.60 ± 6.36 | 3.35 ± 3.12 | 0.50 ± 0.46 | 1.98 ± 1.76 |
| 10 | >50 | >50 | 2.15 ± 0.93 | >50 |
| 11 | >50 | >50 | 0.50 ± 0.35 | >50 |
| VCR | 10.13 nM | 0.23 nM | 0.04 nM | 0.30 nM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Chen, W.-T.; Li, S.-W.; Ye, J.-Y.; Huan, X.-J.; Gavagnin, M.; Yao, L.-G.; Wang, H.; Miao, Z.-H.; Li, X.-W.; et al. Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and Their Sponge-Prey Acanthella cavernosa. Mar. Drugs 2019, 17, 56. https://doi.org/10.3390/md17010056
Wu Q, Chen W-T, Li S-W, Ye J-Y, Huan X-J, Gavagnin M, Yao L-G, Wang H, Miao Z-H, Li X-W, et al. Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and Their Sponge-Prey Acanthella cavernosa. Marine Drugs. 2019; 17(1):56. https://doi.org/10.3390/md17010056
Chicago/Turabian StyleWu, Qihao, Wen-Ting Chen, Song-Wei Li, Jian-Yu Ye, Xia-Juan Huan, Margherita Gavagnin, Li-Gong Yao, Hong Wang, Ze-Hong Miao, Xu-Wen Li, and et al. 2019. "Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia coelestis, and Their Sponge-Prey Acanthella cavernosa" Marine Drugs 17, no. 1: 56. https://doi.org/10.3390/md17010056