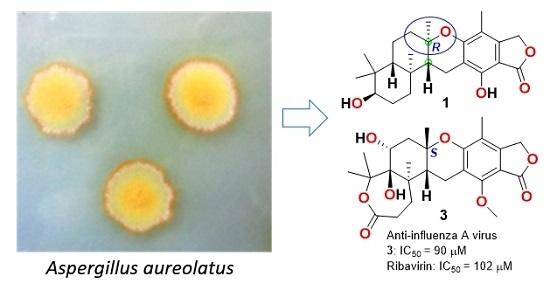
1. Introduction
The austalides are a family of meroterpenoid produced mainly by marine-derived
Aspergillus and
Penicullium genera, 28 members have been reported since the first one discovered in 1984 [
1,
2,
3,
4,
5]. Biosynthetically, they are derived from 6-[(2
E,6
E)farnesyl]-5,7-dihydroxy-4-methylphthalide [
5], followed by cyclization and oxidative modification. Austalides possess diverse structures, which could be divided into four subfamilies corresponding to ring systems including 5/6/6/6 tetracyclic, 5/6/6/6/6 pentacyclic, 5/6/6/6/7 pentacyclic and 5/6/6/6/5/6 hexacyclic rings. Among the austalide meroterpenoids, those containing 5/6/6/6/6 pentacyclic ring system are rare, with only three cases reported including austalides K and L [
2], and 17
S-dihydroaustalide K [
5]. In addition, the terpene rings are fused to the chroman ring in
cis configuration with 11
S, 21
R for most of the austalides. Although the structures are attractive, only limited bioactivities for few of them have been reported such as antibacterial and
endo-1,3-β-
d-glucanase inhibitory activities [
4,
5].
As part of our continuing efforts to discover bioactive products from sponge-derived microorganisms [
6,
7,
8,
9,
10,
11,
12], an
Aspergillus aureolatus strain HDN14-107, isolated from an unidentified sponge collected at Xisha Island, China, was investigated which resulted in the discovery of three new meroterpenoids, named austalides S-U (
1–
3), together with eleven known ones (
4–
14) (
Figure 1). The structures of new compounds were identified by NMR and HRESIMS, and the absolute configurations were determined by comparison of the experimental ECD spectra as well as the time-dependent density functional theory electronic circular dichroism (TDDFT ECD) calculations. Among them, compound
1 is the first austalide with the terpene ring fused to the chroman ring in
trans configuration and also is the fourth case of analogues with 5/6/6/6/6 pentacyclic ring system. Compounds
3 and
5 exhibited anti-influenza virus A (H1N1) activity with IC
50 values of 90 and 99 μM, respectively. Herein, we report the isolation, structure elucidation and bioactivities of the new compounds.
2. Results and Discussion
The molecular formula of austalide S (
1) was determined as C
24H
32O
5 according to the protonated HRESIMS peak at
m/
z 401.2323 (
Supplementary Figure S1). The
1H and
13C NMR (
Table 1 and
Table 2) spectra indicated the presence of five methyls, appearing as singlet in the
1H NMR spectrum, including one aromatic methyl group at
δH 2.05 ppm (CH
3-23), and four aliphatic ones at
δH 1.34, 0.99, 0.96 and 0.78 ppm. Additionally, six methylenes with one oxygenated one at
δH 5.12 (C-1), three methines, and ten non-protonated carbons were observed. A comparison of the 1D NMR data (
Table 1 and
Table 2) with those for 17
S-dihydroaustalide K [
5] showed high similarity. The key differences were the replacement of the methoxy group in 17
S-dihydroaustalide K by a hydroxy group (5-OH) in
1, which was further confirmed by the COSY and HMBC correlations (
Figure 2). Although the planar structures of
1 and 17
S-dihydroaustalide K are highly similar, the chemical shifts of C-11, C-12 and C-24 are significantly different (
δC 77.2, 43.6 and 24.7 in
1 vs. 76.5, 40.4 and 27.3 in 17
S-dihydroaustalide K, respectively), indicating that their stereochemistry might be different.
The relative configuration of
1 was deduced from analysis of the NOESY and NOE correlations (
Figure 3). The NOEs observed between CH
3-27 and CH
3-24 indicated that they faced to the same side of ring D. The NOESY correlations of H-14/H-21/H-19 suggested that they oriented to the opposite side of ring D. Due to the chair conformation of ring E, the position of 17-OH group was deduced as axial and on the same face with H-14 based on the NOESY correlations between H-17 and H-25/H-26 which indicated the equatorial position of H-17. Thus, the relative stereochemistry of
1 was established as (11
R*, 14
R*, 17
R*, 20
S*, 21
R*).
Given the ECD have been provided to be a straightforward way for the configurational assignment of austalides [
3], the TDDFT ECD (time-dependent density functional theory electronic circular dichroism) calculations of alternative solution conformers of
1 were carried out. The calculated ECD curves (
Figure 4) of the (11
R, 14
R, 17
R, 20
S, 21
R)-
1 gave a good agreement with the experimental data of compound
1, indicating that the absolute configuration of
1 was 11
R, 14
R, 17
R, 20
S, 21
R. It is the first case for austalides with 11
R configuration.
Austalide T (
2) was obtained with the molecular formula C
27H
34O
9 based on the protonated peak at
m/
z 503.2276 (
Supplementary Figure S10). The 1D NMR data of
2 were almost identical to those of the known austalide A (
4), except for the disappearance of the methoxyl at C-17 in
4, suggesting that
2 possesses the same skeleton as
4 but with a 17-OH group, which agreed with the 14 amu molecular weight loss. Accordingly, slight upfield shifts were observed for C-17 (
δC = 117.8 ppm) in the
13C NMR spectra of
2, compared to C-17 (
δC = 119.3 ppm) of
4. The planar structure of
2 was also confirmed by COSY and HMBC correlations (
Figure 2). Moreover, the NOE spectrum indicated a (11
S*, 13
R*, 14
R*, 17
S*, 20
R*, 21
R*) relative configuration, in agreement with
4. The absolute configuration of
2 was also inferred to be the same as
4 based on the similarity of their ECD spectra (
Figure 5), which allowed us to determine the absolute configuration of
2 as 11
S, 13
R, 14
R, 17
S, 20
R, 21
R.
The HRESIMS of austalide T (
3) indicated the molecular formula C
25H
32O
8 (
Supplementary Figure S18), with one more oxygen atoms than austalide J (
9). Their
1H and
13C NMR data (
Table 1 and
Table 2) revealed that
3 and
9 shared the same pentacyclic skeleton. The differences were attributed to the appearance of a C-14 hydroxy group in
9, and two hydroxy groups in
3 which were located at C-13 and C-14, respectively, according to the chemical shifts (
δC 69.1, C-13 and
δC 86.6, C-14 in
3), and the COSY and HMBC correlations (
Figure 2). The relative configuration of
3 was also determined as (11
S*, 13
R*, 14
R*, 20
R*, 21
R*) by interpretation of the NOE correlations. The absolute configuration was further established to be 11
S, 13
R, 14
R, 20
R, 21
R based on the Cotton effects at 270 nm (Δε −4.65), 229 nm (Δε +4.53) and 212 nm (Δε −4.78) which were similar to those of
9 (
Figure 5).
Those known compounds
4–
14 were identified as austalides A, B, D, E, G, I, J, L, P (
4–
7,
11,
8–
9,
12,
14) [
1,
2,
3,
4], 13-
O-deacetyaustalide I (
10) [
5] and austalide P acid (
13) [
5], respectively, by comparison of their spectroscopic and physical data (
1H and
13C NMR, MS, and ECD) with those reported in the literature.
All the compounds showed no cytotoxic activity (IC
50 > 50 μM). The ant-influenza A virus (H1N1) activities of compounds
1–
14 were evaluated by the CPE inhibition assay [
8,
13]. Compounds
3,
5,
8 and
13 showed inhibitory effects with IC
50 values of 90, 99, 131 and 145 μM, respectively, while other compounds were inactive (IC
50 > 200 μM) (ribavirin as positive control, IC
50 102 μM).
Previous biosynthetic studies have revealed a hybrid polyketide pathway and mevalonate pathway for austalides [
1,
2,
3,
4,
5]. By forming an intermediate with carbocation [
14,
15], compounds
1–
3 were proposed to be generated through a pair of epimers with chromene and
trans-chromene moieties (
Scheme 1), following with further modifications.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured with a JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). UV spectra were recorded on a Beckman DU 640 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA). CD spectra were measured on a JASCO J-715 spectropolarimeter (JASCO Corporation). NMR spectra were recorded on JEOL JNMECP 600 (JEOL Ltd., Tokyo, Japan) and Varian-500 (Varian Medical Systems Inc., Palo Alto, CA, USA) spectrometers using TMS as an internal standard. HRESIMS spectra were measured on a Micromass EI-4000 Autospec-Ultima-TOF (Micromass Communication, Inc., Manchester, UK). Semipreparative HPLC was performed using an YMC-Pack ODS-A column (5 μm, 10 × 250 mm, YMC Co., Ltd., Kyoto, Japan). TLC and column chromatography (CC) were performed on plates precoated with silica gel GF254 (10–40 µm) and over silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China), respectively. Size-exclusion chromatography was performed using Sephadex LH-20 (GE Healthcare, Uppsala, Sweden). The seawater was collected from Huiquan bay, Yellow Sea, China.
3.2. Fungal Material
The fungal strain Aspergillus aureolatus HDN14-107 was isolated from an unidentified sponge collected at Xisha Islands, China and was identified by ITS sequence. The ITS1-5.8S-ITS2 rDNA sequence of the fungus HDN14-107 has been submitted to GenBank with the accession number KC589122. A voucher specimen is deposited in our laboratory at −20 °C. The working strain was prepared on potato dextrose agar slants and stored at 4 °C.
3.3. Fermentation and Extraction
The fungus HDN14-107 was cultured under static conditions at 28 °C in 1 L Erlenmeyer flasks containing 300 mL liquid culture medium, composed of glucose (20.0 g/L), poly peptone (5.0 g/L), yeast extract (3.0 g/L), malt extract (3.0 g/L), and naturally-collected seawater (Huiquan bay, Yellow Sea, China), then adjusting the pH to 7.0. After 3 weeks of cultivation, 70 L of whole broth was filtered through cheesecloth to separate the supernatant from the mycelia. The former was extracted three times with EtOAc, while the latter was extracted three times with acetone and concentrated under reduced pressure to afford an aqueous solution, which was extracted three times with EtOAc. Both EtOAc solutions were combined and concentrated under reduced pressure to give the organic extract (20.0 g).
3.4. Purification
The organic extract was subjected to vacuum liquid chromatography over C18 ODS column using a gradient elution with H2O-MeOH to give four fractions (fraction 1-fraction 4). Fraction 1 was subjected to Sephadex LH-20 (GE Healthcare, Uppsala, Sweden) column chromatography eluting with CH2Cl2-MeOH (1:1), and then purified by a semi-preparative RPHPLC column (55:45 MeOH-H2O, 4 mL/min, YMC Co., Ltd.) to provide compound 3 (43 mg, tR 9.4 min). Fraction 2 was chromatographed by RPMPLC eluting with MeOH-H2O (40%~60%, 110 min, 8 mL/min) to afford fraction 2.1 and fraction 2.2. Fraction 2.1 was further separated by Sephadex LH-20 chromatograph eluting with CH2Cl2-MeOH (1:1) and then on a semi-preparative RPHPLC column (60:40 MeOH-H2O, 4 mL/min) to provide compound 10 (5 mg, tR 9.6 min). Fraction 2.2 was further purified by Sephadex LH-20 chromatograph eluting with CH2Cl2-MeOH (1:1), and then purified by semi-preparative RPHPLC column (57:43 MeOH-H2O, 4 mL/min) to provide compound 2 (5 mg, tR 14.7 min) and 8 (7 mg, tR 12 min). Fraction 3 was chromatographed by RPMPLC eluting with MeOH-H2O (40%~100%, 165 min, 8 mL/min) and further purified by Sephadex LH-20 chromatograph eluting with MeOH to afford fraction 3.1, fraction 3.2 and fraction 3.3. Fraction 3.1 was rechromatographed by semi-preparative RPHPLC (60:40 MeOH-H2O, 3 mL/min) to afford compound 11 (35.9 mg, tR 16.0 min), compound 9 (10 mg, tR 18 min), compound 6 and 7 (50 mg, tR 20 min). Fraction 3.2 was further purified by semi-preparative RPHPLC (60:40 MeOH-H2O, 3 mL/min) to afford compound 1 (3 mg, tR 22.4 min). Fraction 3.3 was purified by semi-preparative RPHPLC (70:30 MeOH-H2O, 3 mL/min) to give compound 13 (10 mg, tR 16.3 min), compound 12 (31.2 mg, tR 19.8 min) and fraction 3.3.1. Fraction 3.3.1 was further purified by semi-preparative RPHPLC (65:35 MeOH-H2O, 3 mL/min) to afford compound 5 (10 mg, tR 18 min). Fraction 4 was rechromatographed on semi-preparative RPMPLC eluting with MeOH-H2O (65%~100%, 115 min, 8 mL/min) to give fraction 4.1, and then further purified by Sephadex LH-20 chromatograph eluting with CH2Cl2-MeOH (1:1) afford fraction 4.1.1 and fraction 4.1.2. Fraction 4.1.1 was eluted with MeOH-H2O (65:35 MeOH-H2O, 3 mL/min) on semi-preparative RPHPLC to provide compound 14 (5.3 mg, tR 24.7 min). Fraction 4.1.2 was purified by semi-preparative RPHPLC (45:55 CH3CN-H2O, 30 min, 3 mL/min) to provide compound 4 (9.6 mg, tR 32 min).
Austalide S (
1): white amorphous powder (MeOH); [
α]
= −22 (
c 0.15, CHCl
3); UV (MeOH)
λmax (log
ε) 218 (4.32), 265 (3.40); ECD (7.0 × 10
−4 M, MeCN)
λmax (Δ
ε) 299 nm (+0.53), 229 nm (−1.53), 209 nm (+4.09);
1H NMR and
13C NMR data see
Table 1 and
Table 2; HRESIMS
m/
z 401.2323 [M + H]
+ (calcd for C
24H
33O
5, 401.2323).
Austalide T (
2): white amorphous powder (MeOH); [
α]
= −88 (
c 0.07, CHCl
3); UV (MeOH)
λmax (log
ε) 221 (3.61), 265 (3.52); ECD (5.9 × 10
−4 M, MeCN)
λmax (Δ
ε) 269 nm (−2.91), 229 nm (+2.45), 212 nm (−2.99);
1H NMR and
13C NMR data see
Table 1 and
Table 2; HRESIMS
m/
z 503.2276 [M + H]
+ (calcd for C
27H
35O
9, 503.2276).
Austalide U (
3): white amorphous powder (MeOH); [
α]
= −46 (
c 0.13, CH
2Cl
2); UV (MeOH)
λmax (log
ε) 221 (4.23), 266 (3.91); ECD (6.5 × 10
−4 M, MeCN)
λmax (Δ
ε) 270 nm (−4.65), 229 nm (+4.53), 212 nm (−4.78);
1H NMR and
13C NMR data see
Table 1 and
Table 2; HRESIMS
m/
z 461.2163 [M + H]
+ (calcd. for C
25H
33O
8, 461.2170).
Austalide A (4): white amorphous powder (MeOH); ECD (9.4 × 10−4 M, MeCN) λmax (Δε) 264 nm (−4.83), 228 nm (+3.22), 210 nm (−2.56).
Austalide J (9): white amorphous powder (MeOH), ECD (1.3 × 10−3 M, MeCN) λmax (Δε) 265 nm (−2.69), 229 nm (+3.15), 210 nm (−1.73).
13-O-deacethylaustalide I (10): white amorphous powder (MeOH); ECD (4.5 × 10−4 M, MeCN) λmax (Δε) 268 nm (−1.82), 229 nm (+1.24), 213 nm (−2.54).
3.5. Computation Section
Conformational searches were run employing the “systematic” procedure implemented in Spartan’14 [
16], using MMFF (Merck molecular force field). All MMFF minima were reoptimized with DFT calculations at the B3LYP/6-31+G(d) level using the Gaussian 09 program [
17]. The geometry was optimized starting from various initial conformations, with vibrational frequency calculations confirming the presence of minima. Time-dependent DFT calculations were performed on three lowest-energy conformations (
Supplementary Figure S27) for the configuration using 20 excited states, and using a polarizable continuum model (PCM) for acetonitrile. ECD spectra were generated using the program SpecDis [
18] by applying a Gaussian band shape with 0.20 eV width, from dipole-length rotational strengths. The dipole velocity forms yielded negligible differences.