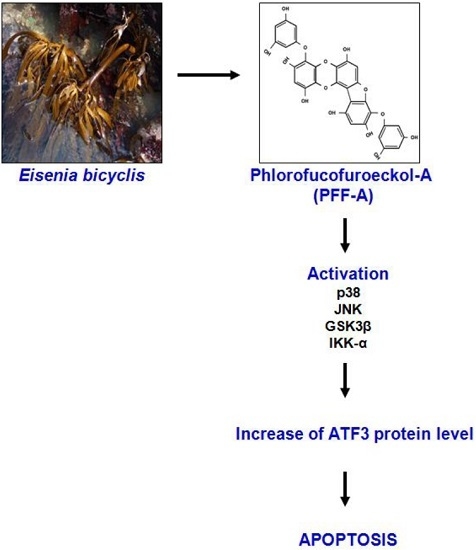
In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of PFF-A on the Viability of Human Colorectal Cancer Cells
2.2. Effect of PFF-A on ATF3 Expression in Human Colorectal Cancer Cells
2.3. Identification of Cis-Acting Element Responsible for PFF-A-Induced ATF3 Activation
2.4. Upstream Kinases Associated with the Increase of ATF3 Expression by PFF-A
2.5. ATF3 Mediates the Induction of Apoptosis by PFF-A
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Cell Death Assay
4.5. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.6. Transient Transfections
4.7. Transfection of Small Interference RNA (siRNA)
4.8. Expression Vector
4.9. SDS-PAGE and Western Blot
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, S.Y.; Hail, N.J.; Lotan, R. Apoptosis as a novel target for cancer chemoprevention. J. Nat. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Costantini, P.; Jocotot, E.; Decaudin, D.; Kroemer, G. Mitochondrion as a novel target of anticancer chemotherapy. J. Nat. Cancer Inst. 2000, 92, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Apoptosis-targeted therapies for cancer. Cancer Cell 2003, 3, 17–22. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. Mechanisms of cancer chemoprevention in hepatic carcinogenesis: Modulation of focal lesion growth in mice. Toxicol. Sci. 1999, 52, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Samaha, H.S.; Kelloff, G.J.; Steele, V.; Rao, C.V.; Reddy, B.S. Modulation of apoptosis by sulindac, curcumin, phenylethy l-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: Apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997, 57, 1301–1305. [Google Scholar] [PubMed]
- Yang, K.; Lamprecht, S.A.; Liu, Y.; Shinozaki, H.; Fan, K.; Leung, D.; Newmark, H.; Steele, V.E.; Kelloff, G.J.; Lipkin, M. Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis 2000, 21, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Sakata, K.; Yamada, Y.; Hirose, Y.; Sugie, S.; Mori, H. Modifying effects of dietary capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis 2002, 23, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Bus, P.J.; Nagtegaal, I.D.; Verspaget, H.W.; Lamers, C.B.; Geldof, H.; van Krieken, J.H.; Griffioen, G. Mesalazine-induced apoptosis of colorectal cancer: On the verge of a new chemopreventive era? Aliment. Pharmacol. Ther. 1999, 13, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Reinacher-Schick, A.; Seidensticker, F.; Petrasch, S.; Reiser, M.; Philippou, S.; Theegarten, D.; Freitag, G.; Schmiegel, W. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy 2000, 32, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wolfgang, C.D.; Chen, B.P.; Chen, T.H.; Hai, T. ATF3 gene. Genomic organization, promoter, and regulation. J. Biol. Chem. 1996, 271, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Soung, N.K.; Thimmegowda, N.R.; Jeong, S.J.; Jang, J.H.; Moon, D.O.; Chung, J.K.; Lee, K.S.; Kwon, Y.T.; Erikson, R.L.; et al. Patulin induces colorectal cancer cells apoptosis through EGR-1 dependent ATF3 up-regulation. Cell. Signal. 2012, 24, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bahn, J.H.; Whitlock, N.C.; Baek, S.J. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene 2010, 29, 5182–5192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yamaguchi, K.; Kim, J.S.; Eling, T.E.; Safe, S.; Park, Y.; Baek, S.J. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis 2006, 27, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, J.; Park, Y.; Lee, S.H. ATF3 Mediates Anti-Cancer Activity of Trans-10, Cis-12-Conjugated Linoleic Acid in Human Colon Cancer Cells. Biomol. Ther. 2015, 23, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Lee, S.H.; Kim, J.S.; Wimalasena, J.; Kitajima, S.; Baek, S.J. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Res. 2006, 66, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Jamaluddin, M.S.; Aggarwal, B.; Myers, J.; Boyd, D.D. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol. Cancer Ther. 2005, 4, 233–241. [Google Scholar] [PubMed]
- Lee, S.H.; Min, K.W.; Zhang, X.; Baek, S.J. 3,3’-diindolylmethane induces activating transcription factor 3 (ATF3) via ATF4 in human colorectal cancer cells. J. Nutr. Biochem. 2013, 24, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Son, B.W.; Choi, J.S. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem. Pharm. Bull. 2003, 51, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-kappaB, Akt, and p38 MAPK. Toxicol. Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Mori, H.; Nakayama, Y.; Takahashi, M. Anti-plasmin inhibitor. VI. Structure of phlorofucofuroeckol A, a novel phlorotannin with both dibenzo-1,4-dioxin and dibenzofuran elements, from Ecklonia kurome Okamura. Chem. Pharm. Bull. 1990, 38, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc epsilonRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- You, H.N.; Lee, H.A.; Park, M.H.; Lee, J.H.; Han, J.S. Phlorofucofuroeckol A isolated from Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2015, 752, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.N.; Sukhthankar, M.; Lee, S.H.; Yoon, J.H.; Baek, S.J. Green tea catechin (−)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. Eur. J. Cancer 2007, 43, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Bottone, F.G., Jr.; Martinez, J.M.; Alston-Mills, B.; Eling, T.E. Gene modulation by Cox-1 and Cox-2 specific inhibitors in human colorectal carcinoma cancer cells. Carcinogenesis 2004, 25, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Lu, D.; Hai, T.; Boyd, D.D. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 2005, 24, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mo, P.; Ren, S.; Yan, C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J. Biol. Chem. 2010, 285, 13201–13210. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wolfgang, C.D.; Hai, T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J. Biol. Chem. 2006, 281, 10473–10481. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Wang, D.L. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol. Pharmacol. 2004, 65, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.; Lang, S.A.; Moser, C.; Mori, A.; Fichtner-Feigl, S.; Hellerbrand, C.; Dietmeier, W.; Schlitt, H.J.; Geissler, E.K.; Stoeltzing, O. Activating transcription factor-3 (ATF3) functions as a tumor suppressor in colon cancer and is up-regulated upon heat-shock protein 90 (Hsp90) inhibition. BMC Cancer 2010, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Iiizumi-Gairani, M.; Okuda, H.; Kobayashi, A.; Watabe, M.; Pai, S.K.; Pandey, P.R.; Xing, F.; Fukuda, K.; Modur, V.; et al. KAI1 gene is engaged in NDRG1 gene-mediated metastasis suppression through the ATF3-NFkappaB complex in human prostate cancer. J. Biol. Chem. 2011, 286, 18949–18959. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.L.; Albanese, C.; Pestell, R.G.; LaMarre, J. Activating transcription factor 3 induces DNA synthesis and expression of cyclin D1 in hepatocytes. J. Biol. Chem. 2001, 276, 27272–27280. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Hua, B.; Adachi, S.; Guney, I.; Kawauchi, J.; Morioka, M.; Tamamori-Adachi, M.; Tanaka, Y.; Nakabeppu, Y.; Sunamori, M.; et al. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 2005, 24, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wolford, C.C.; Chang, Y.S.; McConoughey, S.J.; Ramsey, S.A.; Aderem, A.; Hai, T. ATF3, an adaptive-response gene, enhances TGFβ signaling and cancer-initiating cell features in breast cancer cells. J. Cell. Sci. 2010, 123, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Della Coletta, L.; Powell, K.L.; Shen, J.; Thames, H.; Aldaz, C.M.; MacLeod, M.C. Activation of the canonical Wnt/beta-catenin pathway in ATF3-induced mammary tumors. PLoS ONE 2011, 6, e16515. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Kim, J.S.; Jackson, F.R.; Eling, T.E.; McEntee, M.F.; Lee, S.H. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 2004, 25, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.G.; Lu, D.; Kim, M.L.; Kociba, G.J.; Shukri, T.; Buteau, J.; Wang, X.; Frankel, W.L.; Guttridge, D.; Prentki, M.; et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol. Cell. Biol. 2004, 24, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, B.; Correia, J.S.; Mary, J.L.; Tomás-Zuber, M.; Lesslauer, W. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38alphaMAPK). J. Biol. Chem. 1998, 273, 29661–29671. [Google Scholar] [CrossRef] [PubMed]
- Fiol, C.J.; Williams, J.S.; Chou, C.H.; Wang, Q.M.; Roach, P.J.; Andrisani, O.M. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J. Biol. Chem. 1994, 269, 32187–32193. [Google Scholar] [PubMed]
- Kwon, T.H.; Kim, T.W.; Kim, C.G.; Park, N.H. Antioxidant activity of various solvent fractions from edible brown alga, Eisenia bicyclis and its active compounds. J. Food Sci. 2013, 78, 679–684. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eo, H.J.; Kwon, T.-H.; Park, G.H.; Song, H.M.; Lee, S.-J.; Park, N.-H.; Jeong, J.B. In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells. Mar. Drugs 2016, 14, 69. https://doi.org/10.3390/md14040069
Eo HJ, Kwon T-H, Park GH, Song HM, Lee S-J, Park N-H, Jeong JB. In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells. Marine Drugs. 2016; 14(4):69. https://doi.org/10.3390/md14040069
Chicago/Turabian StyleEo, Hyun Ji, Tae-Hyung Kwon, Gwang Hun Park, Hun Min Song, Su-Jin Lee, Nyun-Ho Park, and Jin Boo Jeong. 2016. "In Vitro Anticancer Activity of Phlorofucofuroeckol A via Upregulation of Activating Transcription Factor 3 against Human Colorectal Cancer Cells" Marine Drugs 14, no. 4: 69. https://doi.org/10.3390/md14040069