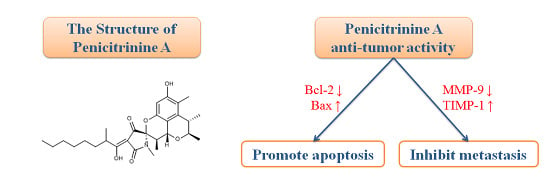
Antitumor Effects and Related Mechanisms of Penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium citrinum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation


| Position | δH (J in Hz) | δC |
|---|---|---|
| 1 | 5.03 (1H, d, 11.0) | 62.9 CH |
| 3 | 4.14 (1H, q, 6.8) | 75.1 CH |
| 4 | 2.62 (1H, q, 6.8) | 34.8 CH |
| 5 | 115.3 qC | |
| 6 | 154.2 qC | |
| 7 | 6.08 (1H, s) | 100.3 CH |
| 8 | 149.2 qC | |
| 9 | 111.4 qC | |
| 10 | 136.9 qC | |
| 11 | 1.40 (3H, d, 6.8) | 18.7 CH3 |
| 12 | 1.21 (3H, d, 6.8) | 22.4 CH3 |
| 13 | 2.05 (3H, s) | 10.1 CH3 |
| 2′ | 173.9 qC | |
| 3′ | 99.9 qC | |
| 4′ | 191.4 qC | |
| 5′ | 94.0 qC | |
| 6′ | 2.29 (1H, dq, 11.0, 6.8) | 35.7 CH |
| 7′ | 0.99 (3H, d, 6.8) | 10.6 CH3 |
| 8′ | 192.3 qC | |
| 9′ | 3.59 (1H, m) | 36.2 CH |
| 10′ | 1.69 (1H, m) | 34.0 CH2 |
| 1.47 (1H, m) | ||
| 11′ | 1.24~1.37 (2H, m) | 27.3 CH2 |
| 12′ | 1.24~1.37 (2H, m) | 29.3 CH2 |
| 13′ | 1.24~1.37 (2H, m) | 22.7 CH2 |
| 14′ | 1.24~1.37 (2H, m) | 31.8 CH2 |
| 15′ | 0.87 (3H, t, 7.0) | 14.2 CH3 |
| 16′ | 1.17 (3H, d, 6.9) | 17.3 CH3 |
| 17′ | 2.90 (3H, s) | 23.1 CH3 |
2.2. Biosynthesis of Penicitrinine A

2.3. Penicitrinine A Inhibits the Proliferation of Multiple Tumor Types

| Human Cancer Type | Cell Line | IC50 (μM) | Human Cancer Type | Cell Line | IC50 (μM) |
|---|---|---|---|---|---|
| Stomach cancer | SGC-7901 | >50 | Liver cancer | PLC/PRF/5 | 46.13 |
| BGC-823 | 44.58 | Huh-7 | 49.87 | ||
| HGC-27 | 29.49 | SK-HEP-1 | 33.20 | ||
| Lung cancer | 95-D | 47.50 | Nasopharynx cancer | CNE-1 | 31.76 |
| SK-MES-1 | 42.40 | CNE-2 | 45.17 | ||
| SPC-A1 | 28.67 | Esophagus cancer | EC-9706 | 36.14 | |
| A-549 | >50 | KYSE450 | 38.89 | ||
| Colon cancer | SW-480 | 41.59 | Breast cancer | SKBR-3 | 41.88 |
| SW-620 | >50 | MCF-7 | >50 | ||
| LOVO | 42.33 | Lymphoma cancer | Jeko-1 | >50 | |
| Malignant melanoma | A-375 | 20.12 | U937 | >50 | |
| Oral epidermoid carcinoma | KB | 35.25 |
2.4. Penicitrinine A Inhibits the Proliferation of A-375 Malignant Melanoma Cells
2.5. Penicitrinine A Induces Significant Apoptotic Morphological Changes in A-375 Cells


2.6. Penicitrinine A Induces Apoptosis in A-375 Cells

2.7. Penicitrinine A Modulates Apoptosis -Related mRNA and Protein in A-375 Cells

2.8. Penicitrinine A Inhibits the Cell Migration and Invasion in A-375 Cells


2.9. Penicitrinine A Modulates Metastatic-Related Proteins in A-375 Cells

2.10. Discussion
3. Experimental Section
3.1. Isolation of Penicitrinine A
3.1.1. General Experimental Procedures
3.1.2. Fungal Material
3.1.3. Fermentation and Extraction
3.1.4. Purification of Penicitrinine A
3.1.5. Spectral Data
3.2. Cell Culture and Treatment
3.3. WST-1 Cell Proliferation Assay
3.4. RTCA Cytotoxicity Assay
3.5. Morphological Analysis
3.5.1. Hoechst 33258 Staining
3.5.2. AO/EB Staining
3.6. Flow Cytometry Analysis
3.7. Wound Healing Assay
3.8. Trans-Well Assay
3.9. Real-Time Quantitative PCR
| Gene | Sequences | |
|---|---|---|
| Bcl-2 | forward | 5′-CGACTTTGCAGAGATGTCCA-3′ |
| reverse | 5′-ATGCCGGTTCAGGTACTCAG-3′ | |
| Bax | forward | 5′-CCTTTTCTACTTTGCCAGCAAAC-3′ |
| reverse | 5′-GAGGCCGTCCCAACCAC-3′ | |
| MMP-9 | forward | 5′-CCTGGAGACCTGAGAAC-3′ |
| reverse | 5′-CAGGGACAGTTGCTTCT-3′ | |
| TIMP-1 | forward | 5′-ACTCTTGCACATCACTACCT-3′ |
| reverse | 5′-AAACACTGTGCATTCCTC-3′ | |
| GAPDH | forward | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| reverse | 5′-GAAGATGGTGATGGGATTTC-3′ |
3.10. Western Blot
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HPLC | high performance liquid chromatography |
| NMR | nuclear magnetic resonance |
| HRESIMS | high resolution electrospray ionization mass spectroscopy |
| DEPT | distortionless enhancement by polarization transfer |
| HMBC | heteronuclear multiple bond connectivity |
| HMQC | heteronuclear multiple quantum connectivity |
| 5-Fu | 5-fluorouracil |
| AO/EB | acridine orange/ethidium bromide |
| MMP-9 | matrix metallopeptidase 9 |
| TIMP-1 | metallopeptidase inhibitor 1 |
| RTCA | real time cell analyzer |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| PD-1 | programmed cell death 1 |
| COSY | (homonuclear chemical shift) correlation spectroscopy |
| NOESY | nuclear overhauser enhancement spectroscopy |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| FBS | fetal bovine serum |
| PI | propidium iodide |
| TMS | tetramethylsilane |
| DMEM | dulbecco’s modified eagle’s medium |
| PBS | phosphate buffered solution |
References
- Landis, S.H.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer statistics, 1999. CA-Cancer J. Clin. 1999, 49, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Rigel, D.S. Epidemiology of melanoma. Semin. Cutan. Med. Surg. 2010, 29, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.-Y.; Zhang, J.-Y.; Liang, Y.-J.; Chen, L.-M.; Zheng, L.-S.; Wang, F.; Mi, Y.-J.; She, Z.-G.; To, K.K.W.; Lin, Y.-C. Anticancer effect and structure-activity analysis of marine products isolated from metabolites of mangrove fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.D.; Carcache de Blanco, E.J.; Chai, H.B.; Orjala, J.; Farnsworth, N.R.; Soejarto, D.D.; Oberlies, N.H.; Wani, M.C.; Kroll, D.J.; Pearce, C.J.; et al. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009, 81, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-Q.; Wang, J.-F.; Hao, Y.-Y.; Wang, Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.L.; Hill, R.T.; Place, A.R.; Hamann, M.T. The expanding role of marine microbes in pharmaceutical development. Curr. Opin. Biotechnol. 2010, 21, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.S.; Nicholson, B.; Teisan, S.; Lam, K.S.; Potts, B.C. Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J. Nat. Prod. 2004, 67, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G. Panning for chemical gold: Marine bacteria as a source of new therapeutics. Trends Biotechnol. 2009, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Lavrard, H.; Salvetti, B.; Mathieu, V.; Rodriguez, F.; Kiss, R.; Delfourne, E. Synthesis and in vitro antiproliferative activity of amido and amino analogues of the marine alkaloid isogranulatimide. Chem. Med. Chem. 2015, 10, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.M.; de Lima, A.B.; da Silva Barbosa, M.C.; de Camargos, L.F.; de Oliveira, J.T.; de Souza Barbosa, C.; Villar, J.A.; Costa, A.C.; da Silva, I.V.G.; Silva, L.M.; et al. Synthesis and biological evaluation of novel 3-alkylpyridine marine alkaloid analogs with promising anticancer activity. Mar. Drugs 2014, 12, 4361–4378. [Google Scholar] [CrossRef] [PubMed]
- Demchuk, D.V.; Samet, A.V.; Chernysheva, N.B.; Ushkarov, V.I.; Stashina, G.A.; Konyushkin, L.D.; Raihstat, M.M.; Firgang, S.I.; Philchenkov, A.A.; Zavelevich, M.P.; et al. Synthesis and antiproliferative activity of conformationally restricted 1,2,3-triazole analogues of combretastatins in the seaurchin embryo model and agaist human cancel cell lines. Bioog. Med. Chem. 2014, 22, 738–755. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Barraja, P.; Spanò, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.-H. Synthesis and antiproliferative activity of 2,5-bis(3′-Indolyl)pyrroles, analogues of the marine alkaloid Nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; de Cesare, M.; et al. Novel 1H-pyrrolo[2,3-b]pyridine derivatives nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b]pyridines, nortopsentin analogues with antiproliferative activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Liu, W.; Hu, X.; Huang, K.; Wu, J.-L.; Zhang, Q.-Q. Citrinin derivatives from the marine-derived fungus Penicillium citrinum. Chem. Pharm. Bull. 2011, 59, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Lu, Z.-Y.; Zhu, T.-J.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem. Pharm. Bull. 2008, 56, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, W.; Huang, K.; Hu, X.; Fang, Z.-X.; Wu, J.-L.; Zhang, Q.-Q. Penicitrinols F–I, new citrinin derivatives from the marine-derived fungus Penicillium citrinum. Heterocycles 2011, 83, 1853–1858. [Google Scholar] [CrossRef]
- Chen, L.; Huang, K.; Zhong, P.; Hu, X.; Fang, Z.-X.; Wu, J.-L.; Zhang, Q.-Q. Tumonoic acids K and L, novel metabolites from the marine-derived fungus Penicillium citrinum. Heterocycles 2012, 85, 413–419. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Essack, M.; Bajic, V.B.; Archer, J.A. Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar. Drugs 2011, 9, 1580–1606. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gong, M.-W.; Peng, Z.-F.; Zhou, T.; Ying, M.-G.; Zheng, Q.-H.; Liu, Q.-Y.; Zhang, Q.-Q. The marine fungal metabolite, dicitrinone B, induces A375 cell apoptosis through the ROS-related caspase pathway. Mar. Drugs 2014, 12, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, A.; Goping, G.; Hirszel, P. Gliotoxin-induced cytotoxicity proceeds via apoptosis and is mediated by caspases and reactive oxygen species in LLC-PK1 cells. Toxicol. Sci. 2000, 54, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Lee, J.S.; Qian, Z.J.; Li, Y.X.; Kim, K.N.; Heo, S.J.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Je, J.Y.; et al. Gliotoxin isolated from marine fungus Aspergillus sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar. Drugs 2014, 12, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari Tregno, F.; Sau, A.; Pezzola, S.; Geroni, C.; Lapenta, C.; Spada, M.; Filomeni, G.; Bonanno, E.; Federici, G.; Caccuri, A.M. In vitro and in vivo efficacy of 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX) on human melanoma. Eur. J. Cancer 2009, 45, 2606–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-H.; Su, J.-H.; Chiu, C.-C.; Lin, J.-J.; Yang, Z.-Y.; Hwang, W.-I.; Chen, Y.-K.; Lo, Y.-H.; Wu, Y.-J. Proteomic investigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar. Drugs 2013, 11, 2625–2642. [Google Scholar] [CrossRef] [PubMed]
- Denicourt, C.; Dowdy, S.F. Medicine. Targeting apoptotic pathways in cancer cells. Science 2004, 305, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Chang, J.-T.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J.; et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann. Surg. Oncol. 2008, 15, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl-2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Ahonen, M.; Baker, A.H.; Kahari, V.M. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998, 58, 2310–2315. [Google Scholar] [PubMed]
- Buettner, R.; Mesa, T.; Vultur, A.; Lee, F.; Jove, R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol. Cancer Res. 2008, 6, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Baritaki, S.; Militello, L.; Malaponte, G.; Bevelacqua, Y.; Bonavida, B. The role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-kappaB/Snail/RKIP/PTEN circuit. Genes Cancer 2010, 1, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Singh, T.; Katiyar, S.K. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS ONE 2011, 6, e21539. [Google Scholar] [CrossRef] [PubMed]
- Caramel, J.; Papadogeorgakis, E.; Hill, L.; Browne, G.J.; Richard, G.; Wierinckx, A.; Saldanha, G.; Osborne, J.; Hutchinson, P.; Tse, G.; et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 2013, 24, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yan, H.; Zhou, Q. AG1478 inhibits the migration and invasion of cisplatin-resistant human lung adenocarcinoma cells via the cell cycle regulation by matrix metalloproteinase-9. Oncol. Lett. 2014, 8, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Li, R.; Zeng, G.-F.; Liu, B.; Liu, J.; Shu, Y.; Liu, Z.-K.; Qiu, Z.-D.; Wang, D.-J.; Miao, H.-L.; et al. Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression. World J. Gastroenterol. 2014, 20, 10082–10093. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-Y.; Zhou, T.; Zhao, Y.-Y.; Chen, L.; Gong, M.-W.; Xia, Q.-W.; Ying, M.-G.; Zheng, Q.-H.; Zhang, Q.-Q. Antitumor Effects and Related Mechanisms of Penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium citrinum. Mar. Drugs 2015, 13, 4733-4753. https://doi.org/10.3390/md13084733
Liu Q-Y, Zhou T, Zhao Y-Y, Chen L, Gong M-W, Xia Q-W, Ying M-G, Zheng Q-H, Zhang Q-Q. Antitumor Effects and Related Mechanisms of Penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium citrinum. Marine Drugs. 2015; 13(8):4733-4753. https://doi.org/10.3390/md13084733
Chicago/Turabian StyleLiu, Qin-Ying, Tong Zhou, Yang-Yang Zhao, Li Chen, Mei-Wei Gong, Qi-Wen Xia, Min-Gang Ying, Qiu-Hong Zheng, and Qi-Qing Zhang. 2015. "Antitumor Effects and Related Mechanisms of Penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium citrinum" Marine Drugs 13, no. 8: 4733-4753. https://doi.org/10.3390/md13084733