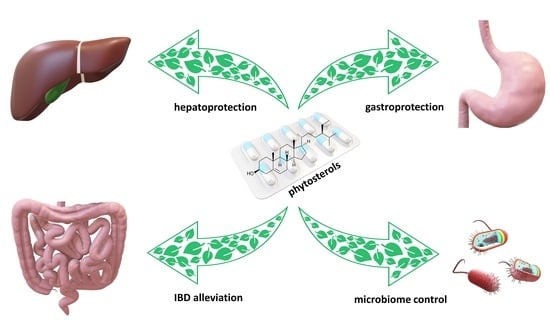
Phytosterols and the Digestive System: A Review Study from Insights into Their Potential Health Benefits and Safety
Abstract
:1. Introduction
2. Chemical Properties of Phytosterols
Absorption, Distribution and Excretion of Phytosterols
3. Phytosterol-Associated Abnormalities
3.1. Phytosterolemia
3.2. Intestinal Failure-Associated Liver Disease (IFALD)
3.3. Interactions in Absorption of Vitamins
4. Health-Promoting Effects of Phytosterols
4.1. Anti-Inflammatory Properties
4.1.1. Anti-Ulcerogenic
4.1.2. Inflammatory Bowel Disease Benefits
4.2. Hepatoprotective Properties
4.2.1. Non-Alcoholic Fatty Liver Disease
4.2.2. Xenobiotics Causing Liver Damage
Carbon Tetrachloride
Paracetamol
Alcohol-Induced Hepatotoxicity
4.3. Anti-Cancer Properties
4.4. Impact of Gut Microbiota
5. Conclusions
6. Methods of Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC transporter | ATP-Binding Cassette transporter |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CCl4 | carbon tetrachloride |
| COX | cyclooxygenases |
| DSS | dextran sulfate sodium |
| HDL | high-density lipoprotein |
| IBD | inflammatory bowel disease |
| IFALD | intestinal failure-associated liver disease |
| LDL | low-density lipoprotein |
| MAPK | mitogen-activated protein kinases |
| NAFLD | non-alcoholic fatty liver disease |
| NSAID | non-steroidal anti-inflammatory drug |
| ROS | reactive oxygen species |
| VLDL | very-low-density lipoprotein |
References
- De Bruyne, L.; Höfte, M.; De Vleesschauwer, D. Connecting growth and defense: The emerging roles of brassinosteroids and gibberellins in plant innate immunity. Mol. Plant 2014, 7, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Amiot, M.J.; Knol, D.; Cardinault, N.; Nowicki, M.; Bott, R.; Antona, C.; Borel, P.; Bernard, J.-P.; Duchateau, G.; Lairon, D. Phytosterol ester processing in the small intestine: Impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans. J. Lipid Res. 2011, 52, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Çağlar, M.Y.; Demirci, M.; Bayrambaş, K.; Çakır, B.; Gülseren, İ. Nanoencapsulation of enzymes, bioactive peptides, and biological molecules. In Nanoencapsulation of Food Bioactive Ingredients. Principles and Applications; Jafari, S.M., Ed.; Academic Press: London, UK, 2017; pp. 297–332. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.M.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Buscail, C.; Sabate, J.-M.; Bouchoucha, M.; Kesse-Guyot, E.; Hercberg, S.; Benamouzig, R.; Julia, C. Western dietary pattern is associated with irritable bowel syndrome in the French nutrinet cohort. Nutrients 2017, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; He, F.; Karin, M. From liver fat to cancer: Perils of the Western diet. Cancers 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lesbros-Pantoflickova, D.; Michetti, P.; Fried, M.; Beglinger, C.; Blum, A.L. Meta-analysis: The treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004, 20, 1253–1269. [Google Scholar] [CrossRef]
- Bouic, P.J.D. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Nguyen, T.T. The cholesterol-lowering action of plant stanol esters. J. Nutr. 1999, 129, 2109–2112. [Google Scholar] [CrossRef]
- Fahy, D.M.; O’Callaghan, Y.C.; O’Brien, N.M. Phytosterols: Lack of cytotoxicity but interference with β-carotene uptake in Caco-2 cells in culture. Food Addit. Contam. 2004, 21, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.H.; Rideout, T.C. Plant sterols: Nutritional aspects. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, ON, Canada, 2011; pp. 535–542. [Google Scholar] [CrossRef]
- Han, S.; Jiao, J.; Xu, J.; Zimmermann, D.; Actis-Goretta, L.; Guan, L.; Zhao, Y.; Qin, L. Effects of plant stanol or sterol-enriched diets on lipid profiles in patients treated with statins: Systematic review and meta-analysis. Sci. Rep. 2016, 6, 31337. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, B. A phytosterol-enriched spread improves lipid profile and insulin resistance of women with gestational diabetes mellitus: A randomized, placebo-controlled double-blind clinical trial. Diabetes Technol. Ther. 2016, 18, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Ivanova, E.A. Antiatherosclerotic efficacy of nutraceuticals. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 61–73. [Google Scholar] [CrossRef]
- Law, M. Plant sterol and stanol margarines and health. BMJ 2000, 320, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.-G. Sitosterolemia: A review and update of pathophysiology, clinical spectrum, diagnosis, and management. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 7–14. [Google Scholar] [CrossRef]
- Berge, K.E.; Tian, H.; Graf, G.A.; Yu, L.; Grishin, N.V.; Schultz, J.; Kwiterovich, P.; Shan, B.; Barnes, R.; Hobbs, H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000, 290, 1771–1775. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, cholesterol control, and cardiovascular disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.K.; Connor, W.E. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Investig. 1974, 53, 1033–1043. [Google Scholar] [CrossRef]
- James, W.D.; Elston, D.M.; McMahon, P.J. Errors in metabolism. In Andrews’ Diseases of the Skin Clinical Atlas; James, W.D., Elston, D.M., McMahon, P.J., Eds.; Elsevier: Edinburgh, UK, 2018; pp. 361–378. [Google Scholar] [CrossRef]
- Salen, G.; Shefer, S.; Nguyen, L.; Ness, G.C.; Tint, G.S.; Shore, V. Sitosterolemia. J. Lipid Res. 1992, 33, 945–955. [Google Scholar] [CrossRef]
- Marín-García, J. CHAPTER 8—Molecular basis of lipoprotein disorders, atherogenesis, and thrombosis. In Post-Genomic Cardiology; Marín-García, J., Ed.; Academic Press: Burlington, ON, Canada, 2007; pp. 211–260. [Google Scholar] [CrossRef]
- Tsubakio-Yamamoto, K.; Nishida, M.; Nakagawa-Toyama, Y.; Masuda, D.; Ohama, T.; Yamashita, S. Current therapy for patients with sitosterolemia –effect of Ezetimibe on plant sterol metabolism. J. Atheroscler. Thromb. 2010, 17, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Allardyce, D.B. Cholestasis caused by lipid emulsions. Surg. Gynecol. Obstet. 1982, 154, 641–647. [Google Scholar] [PubMed]
- Nghiem-Rao, T.H. Potential hepatotoxicities of intravenous fat emulsions in infants and children. Nutr. Clin. Pract. 2016, 31, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.-F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Hendriks, H.F.J.; Brink, E.J.; Meijer, G.W.; Princen, H.M.G.; Ntanios, F.Y. Safety of long-term consumption of plant sterol esters-enriched spread. Eur. J. Clin. Nutr. 2003, 57, 681–692. [Google Scholar] [CrossRef]
- Tovey, F.I. Role of dietary phospholipids and phytosterols in protection against peptic ulceration as shown by experiments on rats. World J. Gastroenterol. 2015, 21, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.A.; Akinloye, D.I.; Onigbinde, S.B.; Metibemu, D.S. Phytosterols demonstrate selective inhibition of COX-2: In-vivo and in-silico studies of Nicotiana tabacum. Bioorg. Chem. 2020, 102, 104037. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, Z.; Jiu, M.; You, J.; Xiao, R. The antigastroulcerative activity of beta-sitosterol-beta-D-glucoside and its aglycone in rats. J. West China Univ. Med. Sci. 1992, 23, 98–101. [Google Scholar]
- Jayaraj, A.P.; Tovey, F.I.; Clark, C.G. Possible dietary protective factors in relation to the distribution of duodenal ulcer in India and Bangladesh. Gut 1980, 21, 1068–1076. [Google Scholar] [CrossRef]
- Tovey, F.I.; Capanoglu, D.; Langley, G.J.; Herniman, J.M.; Bor, S.; Ozutemiz, O.; Hobsley, M.; Bardhan, K.D.; Linclau, B. Dietary phytosterols protective against peptic ulceration. Gastroenterol. Res. 2011, 4, 149–156. [Google Scholar] [CrossRef]
- Jainu, M.; Devi, C.S.S. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: Role of proinflammatory cytokines and oxidative damage. Chem.-Biol. Interact. 2006, 161, 262–270. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Alshahrani, A.; Ali, A. Pre-clinical safety and efficacy evaluation of a herbal nanoemulsion-based formulation for treating inflammatory bowel disease. J. AOAC Int. 2022, 105, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, N.; Amato, J.; Pagano, B.; Di Porzio, A.; Micucci, M.; Bolelli, L.; Aldini, R.; Novellino, E.; Budriesi, R.; Randazzo, A. Impact of phytosterols on liver and distal colon metabolome in experimental murine colitis model: An explorative study. J. Enzym. Inhib. Med. Chem. 2019, 34, 1041–1050. [Google Scholar] [CrossRef]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef]
- Bian, Z.; Qin, Y.; Li, L.; Su, L.; Fei, C.; Li, Y.; Hu, M.; Chen, X.; Zhang, W.; Mao, C.; et al. Schisandra chinensis (Turcz.) Baill. Protects against DSS-induced colitis in mice: Involvement of TLR4/NF-κB/NLRP3 inflammasome pathway and gut microbiota. J. Ethnopharmacol. 2022, 298, 115570. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.D.S.; Bonafé, G.A.; Carvalho Santos, J.; Martinez, C.A.R.; Ortega, M.M.; Ribeiro, M.L. The anti-inflammatory properties of licorice (Glycyrrhiza glabra)-derived compounds in intestinal disorders. Int. J. Mol. Sci. 2022, 23, 4121. [Google Scholar] [CrossRef]
- Mahmoud, T.N.; El-Maadawy, W.H.; Kandil, Z.A.; Khalil, H.; El-fiky, N.M.; El Alfy, T.S.M.A. Canna x generalis L.H. Bailey rhizome extract ameliorates dextran sulphate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/NF-κB and NLRP3 inflammasome pathways. J. Ethnopharmacol. 2021, 269, 113670. [Google Scholar] [CrossRef]
- Te Velde, A.A.; Brüll, F.; Heinsbroek, S.E.M.; Meijer, S.L.; Lütjohann, D.; Vreugdenhil, A.; Plat, J. Effects of dietary plant sterols and stanol esters with low- and high-fat diets in chronic and acute models for experimental colitis. Nutrients 2015, 7, 8518–8531. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165–1229. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific roles of lipoxygenases in development and responses to stress in plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Iser, D.; Ryan, M. Fatty liver disease. A practical guide for GPs. Aust. Fam. Physician 2013, 42, 444–447. [Google Scholar]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.J.; Walenbergh, S.M.A.; van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.E.; Guichot, Y.D.; et al. Protective role of plant sterol and stanol esters in liver inflammation: Insights from mice and humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef]
- Devaraj, E.; Roy, A.; Royapuram Veeraragavan, G.; Magesh, A.; Varikalam Sleeba, A.; Arivarasu, L.; Marimuthu Parasuraman, B. β-sitosterol attenuates carbon tetrachloride–induced oxidative stress and chronic liver injury in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Fayed, M.A.A.; Helal, D.; Ahmed, K.A. Assessment of the hepatoprotective effect of developed lipid-polymer hybrid nanoparticles (LPHNPs) encapsulating naturally extracted β-sitosterol against CCl4 induced hepatotoxicity in rats. Sci. Rep. 2019, 9, 19779. [Google Scholar] [CrossRef]
- Acetaminophen. Health Canada. 2023. Available online: https://www.canada.ca/en/health-canada/services/drugs-medical-devices/acetaminophen.html (accessed on 5 September 2023).
- McCrae, J.C.; Morrison, E.E.; MacIntyre, I.M.; Dear, J.W.; Webb, D.J. Long-term adverse effects of paracetamol—A review. Br. J. Clin. Pharmacol. 2018, 84, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Daly, F.F.S.; Fountain, J.S.; Murray, L.; Graudins, A.; Buckley, N.A. Guidelines for the management of paracetamol poisoning in Australia and New Zealand—Explanation and elaboration. Med. J. Aust. 2008, 188, 296–302. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, A.; Rehman, N.U.; Halim, S.A.; Khan, H.; Khan, I.; Csuk, R.; Al-Rawahi, A.; Al-Hatmi, S.; Al-Harrasi, A. Lophenol and lathosterol from resin of Commiphora kua possess hepatoprotective effects in vivo. J. Ethnopharmacol. 2020, 252, 112558. [Google Scholar] [CrossRef]
- Parameswari, S.A.; Chetty, C.M.; Chandrasekhar, K.B. Hepatoprotective activity of Ficus religiosa leaves against isoniazid+rifampicin and paracetamol induced hepatotoxicity. Pharmacogn. Res. 2013, 5, 271–276. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zhang, F.; Wang, M.; Zha, Y.; Zhou, J.; Han, J.; Zhang, S. Daucosterol alleviates alcohol-induced hepatic injury and inflammation through P38/NF-kB/NLRP3 inflammasome pathway. Nutrients 2023, 15, 223. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, A.; Jin, H.; Liu, F. β-Sitosterol attenuates liver injury in a rat model of chronic alcohol intake. Arch. Pharm. Res. 2020, 43, 1197–1206. [Google Scholar] [CrossRef]
- Hamada, K.; Wang, P.; Xia, Y.; Yan, N.; Takahashi, S.; Krausz, K.W.; Hao, H.; Yan, T.; Gonzalez, F.J. Withaferin A alleviates ethanol-induced liver injury by inhibiting hepatic lipogenesis. Food Chem. Toxicol. 2022, 160, 112807. [Google Scholar] [CrossRef]
- Llaverias, G.; Escolà-Gil, J.C.; Lerma, E.; Julve, J.; Pons, C.; Cabré, A.; Cofán, M.; Ros, E.; Sánchez-Quesada, J.L.; Blanco-Vaca, F. Phytosterols inhibit the tumor growth and lipoprotein oxidizability induced by a high-fat diet in mice with inherited breast cancer. J. Nutr. Biochem. 2013, 24, 39–48. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2021, 36, 299–322. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef]
- Baskar, A.A.; Ignacimuthu, S.; Paulraj, G.M.; Al Numair, K.S. Chemopreventive potential of beta-sitosterol in experimental colon cancer model-an in vitro and in vivo study. BMC Complement. Altern. Med. 2010, 10, 24. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Antiproliferative effect of plant sterols at colonic concentrations on Caco-2 cells. J. Funct. Food. 2017, 39, 84–90. [Google Scholar] [CrossRef]
- Álvarez-Sala, A.; Ávila-Gálvez, M.Á.; Cilla, A.; Barberá, R.; Garcia-Llatas, G.; Espín, J.C.; González-Sarrías, A. Physiological concentrations of phytosterols enhance the apoptotic effects of 5-fluorouracil in colon cancer cells. J. Funct. Food. 2018, 49, 52–60. [Google Scholar] [CrossRef]
- Vats, S. Methods for extractions of value-added nutraceuticals from lignocellulosic wastes and their health application. In Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2017; pp. 1–64. [Google Scholar] [CrossRef]
- Normén, A.L.; Brants, H.A.M.; Voorrips, L.E.; Andersson, H.A.; van den Brandt, P.A.; Goldbohm, R.A. Plant sterol intakes and colorectal cancer risk in the Netherlands cohort study on diet and cancer. Am. J. Clin. Nutr. 2001, 74, 141–148. [Google Scholar] [CrossRef]
- Cuevas-Tena, M.; Gómez del Pulgar, E.M.; Benítez-Páez, A.; Sanz, Y.; Alegría, A.; Lagarda, M.J. Plant sterols and human gut microbiota relationship: An in vitro colonic fermentation study. J. Funct. Food. 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, N.; Khan, W.; Md, S.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.-J.; Huang, J.-Y.; Lin, J.; Ma, Y.-M.; He, S.-Q.; Zhang, Y.-W.; Wang, T.-Z.; Cheng, K.; Xiong, Y.; Sun, F.-G.; et al. Phytosterols alleviate hyperlipidemia by regulating gut microbiota and cholesterol metabolism in mice. Oxid. Med. Cell. Longev. 2023, 2023, 6409385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, J.; Zhu, Y.; Deng, B.; Liu, Y.; Wang, S.; Hou, T.; Song, T. Phytosterols protect against osteoporosis by regulating gut microbiota. J. Agric. Food Chem. 2023, 71, 14539–14549. [Google Scholar] [CrossRef]
- Cuevas-Tena, M.; Alegria, A.; Lagarda, M.J.; Venema, K. Impact of plant sterols enrichment dose on gut microbiota from lean and obese subjects using TIM-2 in vitro fermentation model. J. Funct. Food. 2019, 54, 164–174. [Google Scholar] [CrossRef]
- Manoppo, J.I.C.; Nurkolis, F.; Gunawan, W.B.; Limen, G.A.; Rompies, R.; Heroanto, J.P.; Natanael, H.; Phan, S.; Tanjaya, K. Functional sterol improves breast milk quality by modulating the gut microbiota: A proposed opinion for breastfeeding mothers. Front Nutr. 2022, 9, 1018153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Model | Compound/Extract | Respective Mechanisms of Action/Effect | References |
|---|---|---|---|
| in vitro Caco-2 cell line | β-sitosterol | Directing to apoptosis pathway | [66] |
| in vivo Rats model | Phytosterol Nicotiana tabacum extract containing phytosterols | Lowering level and action of COX-2, regeneration of gastrointestinal mucosa and soothing of inflammation inhibiting mainly one isomer of COX in contrast to NSAIDs, promoting liver’s antioxidant defence | [32] |
| Hippophae rhamnoides extract rich in β-sitosterol and its glucosides | Healing stomach and duodenal ulcers | [33] | |
| Cissus quadrangularis extract containing β-sitosterol and ketosterol | During NSAIDs therapy protecting gastric mucosa from free radicals | [36] | |
| Dolichos biflorus extract rich in stigmasterol and β-sitosterol | Preventing and treating gastroduodenal ulcers | [31] | |
| β-sitosterol | Hepatoprotective properties even after exposure on CCl4, anticancer properties without damage of healthy tissues | [10,51] | |
| Phytosterol enriched soybeans and sesame | Elevated antioxidant properties in liver damage caused by paracetamol | [45] | |
| Methanolic extract of Ficus religiosa | Lower increase of transaminase activity and higher glutathione level after liver damage caused by paracetamol, isoniazid and rifampicin | [56] | |
| β-sitosterol isolated from Artemisia | Increase level of glutathione, reducing level of ROS and inflammation process, decreased production of malondialdehyde | [59] | |
| Mice model | Phytosterol extract from Canna, Schisandra chinensis and Glycyrrhiza glabra | Reduce oxidative stress, onset of colitis, relieving symptoms of IBD | [41,42,43] |
| Withafterin A | Lowered expression of the lipogenesis genes, reducing liver lipid accumulation | [60] | |
| Commiphora kua extract rich in lophenol and lathosterol | Tissue regeneration paracetamol induced liver damage | [55] | |
| Sanchezia speciosa extract rich in daucosterol | Upregulates the synthesis of antioxidant agents like catalase and superoxidase dismutase, elevated level of chaperone, upregulation of synthesis of the superoxide dismutase genes, i.e., SOD1 and SOD2, soothing of inflammation process | [58] | |
| Clinical data Colorectal cancer | Phytosterols | Prevents from developing of cancer | [62,70,71] |
| IFALD | Stigmasterol | Inhibits farnesoid X receptor responsible for developing IFALD | [28] |
| The Cohort Study on Diet and Cancer | Phytosterols | No significant data about preventing from colorectal cancer | [10,68] |
| NAFLD | Stigmasterol and β-sitosterol | Reduces the level of liver cholesterol and increases the levels of polyunsaturated fatty acid, reduces level of proinflammatory cytokines, improves histological picture | [49] |
| Colorectal cancer | Stigmasterol and campesterol | Data presenting that high intake of them can lead to colorectal cancer | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszczuk, E.; Bajguz, A.; Kiraga, Ł.; Crowley, K.; Chłopecka, M. Phytosterols and the Digestive System: A Review Study from Insights into Their Potential Health Benefits and Safety. Pharmaceuticals 2024, 17, 557. https://doi.org/10.3390/ph17050557
Miszczuk E, Bajguz A, Kiraga Ł, Crowley K, Chłopecka M. Phytosterols and the Digestive System: A Review Study from Insights into Their Potential Health Benefits and Safety. Pharmaceuticals. 2024; 17(5):557. https://doi.org/10.3390/ph17050557
Chicago/Turabian StyleMiszczuk, Edyta, Andrzej Bajguz, Łukasz Kiraga, Kijan Crowley, and Magdalena Chłopecka. 2024. "Phytosterols and the Digestive System: A Review Study from Insights into Their Potential Health Benefits and Safety" Pharmaceuticals 17, no. 5: 557. https://doi.org/10.3390/ph17050557