Anti-Inflammatory and Anti-Adipocyte Dysfunction Effects of Ficus lindsayana Latex and Root Extracts
Abstract
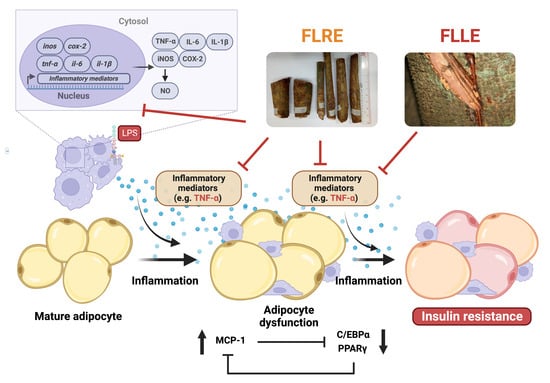
:1. Introduction
2. Results
2.1. Phenolic and Flavonoid Composition of the FLLE and FLRE
2.2. DPPH• and ABTS•+ Scavenging Activities of FLLE and FLRE
2.3. Cytotoxicity of FLLE and FLRE on RAW 264.7 Macrophages, Human PBMCs, Human RBCs, and Mature 3T3-L1 Adipocytes
2.4. Effect of FLLE and FLRE on TNF-α-Induced Insulin Resistance and Adipogenesis in 3T3-L1 Cells
2.5. Effect of FLLE and FLRE on the mRNA Expression of Inflammatory-Associated and Adipogenesis-Related Molecules in TNF-α-Induced 3T3-L1 Adipocytes
2.6. Anti-Inflammatory Activity of FLLE and FLRE in LPS-Stimulated RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Plant Extracts
4.3. Phytochemical Analysis
4.4. Determination of Antioxidant Activities
4.5. Cell Culture
4.6. Cytotoxicity
4.7. Hemolysis Assay
4.8. Adipogenesis
4.9. Glucose Uptake Assay
4.10. Lipolysis Assay
4.11. Measurement of Inflammatory Mediator and Adipogenic mRNA Expressions by Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
4.12. Determination of Nitric Oxide Production
4.13. Enzyme-Linked Immunosorbent (ELISA) Assay
4.14. Western Blotting
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Khanna, R.D.; Karki, K.; Pande, D.; Negi, R.; Khanna, R.S. Inflammation, Free Radical Damage, Oxidative Stress and Cancer. Interdiscip. J. Microinflamm. 2014, 1. [Google Scholar] [CrossRef]
- Hunter, P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Silver, R.; Aldhahi, W.; Cai, D.; Tatro, E.; Lee, J.; Shoelson, S.E. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin. Transl. Sci. 2008, 1, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zavala, M.G.; González-Ortiz, M.; Martínez-Abundis, E.; Robles-Cervantes, J.A.; González-López, R.; Santiago-Hernández, N.J. Effect of Diacerein on Insulin Secretion and Metabolic Control in Drug-Naïve Patients with Type 2 Diabetes: A randomized clinical trial. Diabetes Care 2011, 34, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Osafo, N.; CAgyare; Obiri, D.D.; Antwi, A.O. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); Updated on 1 May 2023. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 30 November 2023).
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech. 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Elisha, I.L.; Dzoyem, J.-P.; McGaw, L.J.; Botha, F.S.; Eloff, J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement. Altern. Med. 2016, 16, 307. [Google Scholar] [CrossRef]
- Ramchoun, M.; Harnafi, H.; Alem, C.; Benlyas, M.; Elrhaffari, L.; Amrani, S. Study on antioxidant and hypolipidemic effects of polyphenol-rich extracts from Thymus vulgaris and Lavendula multifida. Pharmacogn. Res. 2009, 1, 106–112. [Google Scholar]
- Ahmed, F.; Urooj, A. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: A review. Pharm. Biol. 2010, 48, 672–681. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Zhang, B.-D.; Zhu, W.-F.; Zhang, C.-F.; Qin, Y.-M.; Abe, M.; Akihisa, T.; Liu, W.-Y.; Feng, F.; Zhang, J. Traditional uses, phytochemistry, and pharmacology of Ficus hispida L.f.: A review. J. Ethnopharmacol. 2020, 248, 112204. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Mon, A.M.; Fu, Y.; Zhang, Y.; Wang, C.; Yang, X.; Wang, Y. The genus Ficus (Moraceae) used in diet: Its plant diversity, distribution, traditional uses and ethnopharmacological importance. J. Ethnopharmacol. 2018, 226, 185–196. [Google Scholar] [CrossRef]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef]
- Deepa, P.; Sowndhararajan, K.; Kim, S.; Park, S.J. A role of Ficus species in the management of diabetes mellitus: A review. J. Ethnopharmacol. 2018, 215, 210–232. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Khali, M.; Benkhaled, A.; Benamirouche, K.; Baiti, I. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac. J. Trop. Biomed. 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Keshari, A.K.; Kumar, G.; Kushwaha, P.S.; Bhardwaj, M.; Kumar, P.; Rawat, A.; Kumar, D.; Prakash, A.; Ghosh, B.; Saha, S. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J. Ethnopharmacol. 2016, 181, 252–262. [Google Scholar] [CrossRef]
- Olaokun, O.O.; McGaw, L.J.; van Rensburg, I.J.; Eloff, J.N.; Naidoo, V. Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: Comparison of an in vitro assay with an in vivo obese mouse model. BMC Complement. Altern. Med. 2016, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Misbah, H.; Abdul Aziz, A.; Aminudin, N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complement. Altern. Med. 2013, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Jasmine, R.; Manikandan, K.; Karthikeyan, K. Evaluating the antioxidant and anticancer property of Ficus carica fruits. Afr. J. Biotechnol. 2015, 14, 634–641. [Google Scholar] [CrossRef]
- Chandrashekhar, C.; Latha, K.; Vagdevi, H.; Vaidya, V. Anthelmintic activity of the crude extracts of Ficus racemosa. Int. J. Green. Pharm. 2008, 2, 100. [Google Scholar] [CrossRef]
- Beentje, H. Flora of Singapore precursors, 29: A new name for Ficus dubia Wall. ex King (Moraceae). Gard. Bull. Singap. 2021, 73, 307–308. [Google Scholar] [CrossRef]
- Chantarasuwan, B.; Thongaree, S. Five species of Ficus (Moraceae) new for Thailand. Thai For. Bull. 2006, 34, 25–37. [Google Scholar]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Pompimon, W.; Udomputtimekakul, P.; Chaisena, A.; Wattananon, S.; Arsa, A.; Konsantha, K.; Nuntasaen, N. Anti-Syncytium (MC99+ 1A2) and Anti-Bacterial Activities from Twigs and Stems of Ficus dubia. J. Pharm. Res. Int. 2021, 32, 84–91. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Pitchakarn, P.; Ting, P.; Inthachat, W.; Thiyajai, P.; Rodthayoy, D.; Karinchai, J.; Chantarasuwan, B.; Nuchuchua, O.; Temviriyanukul, P. Health-promoting bioactivity and in vivo genotoxicity evaluation of a hemiepiphyte fig, Ficus dubia. Food Sci. Nutr. 2021, 9, 2269–2279. [Google Scholar] [CrossRef]
- Chansriniyom, C.; Nooin, R.; Nuengchamnong, N.; Wongwanakul, R.; Petpiroon, N.; Srinuanchai, W.; Chantarasuwan, B.; Pitchakarn, P.; Temviriyanukul, P.; Nuchuchua, O. Tandem mass spectrometry of aqueous extract from Ficus dubia sap and its cell-based assessments for use as a skin antioxidant. Sci. Rep. 2021, 11, 16899. [Google Scholar] [CrossRef]
- Hu, R.; Chantana, W.; Pitchakarn, P.; Subhawa, S.; Chantarasuwan, B.; Temviriyanukul, P.; Chewonarin, T. Ficus dubia latex extract prevent DMH-induced rat early colorectal carcinogenesis through the regulation of xenobiotic metabolism, inflammation, cell proliferation and apoptosis. Sci. Rep. 2022, 12, 15472. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chantana, W.; Pitchakarn, P.; Subhawa, S.; Chantarasuwan, B.; Temviriyanukul, P.; Chewonarin, T. Ficus dubia latex extract induces cell cycle arrest and apoptosis by regulating the NF-κB pathway in inflammatory human colorectal cancer cell lines. Cancers 2022, 14, 2665. [Google Scholar] [CrossRef]
- Kim, J.-Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Lu, Q.; Li, M.; Zou, Y.; Cao, T. Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity 2014, 22, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Panday, D.R.; Rauniar, G.P. Effect of root-extracts of Ficus benghalensis (Banyan) in memory, anxiety, muscle co-ordination and seizure in animal models. BMC Complement. Altern. Med. 2016, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.M. Protective Effects of latex of Ficus carica L. against lead acetate-induced hepatotoxicity in rats. Jordan. J. Biol. Sci. 2012, 5, 175–182. [Google Scholar]
- Ibraheim, Z.Z.; Nafady, A.M.; Mostafa, M.A.; Amin, F.M. Antioxidant activity and total flavonoids content of aerial parts of Ficus pyriformis Hook. & Arn. (Moraceae) Cultivated in Egypt. Am. J. Chem. 2015, 5, 23–27. [Google Scholar]
- Ali, B.; Mujeeb, M.; Aeri, V.; Mir, S.; Faiyazuddin, M.; Shakeel, F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Nat. Prod. Res. 2012, 26, 460–465. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Cao, H. Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol. 2019, 127, 182–187. [Google Scholar] [CrossRef]
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 Mice through regulation of hepatic lipogenesis gene expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2013, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Neugart, S.; Schreiner, M.; Grune, T.; Rohn, S.; Ott, C. Amaranth’s 2-caffeoylisocitric acid—An anti-inflammatory caffeic acid derivative that impairs NF-κB signaling in LPS-challenged RAW 264.7 macrophages. Nutrients 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-W.; Shen, S.-C.; Wu, J.S.-B. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: Anti-Allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2020, 137, 257–264. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS ONE 2012, 7, e53101. [Google Scholar] [CrossRef]
- Shin, H.-K.; Seo, C.-S.; Jeong, S.-J.; Yoo, S.-R.; Lee, N.-R. Quantitative analysis and in vitro Anti-inflammatory effects of gallic acid, ellagic acid, and quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef]
- Jung, U.J.; Cho, Y.-Y.; Choi, M.-S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Yu, W.; Huang, Y.; Chen, J.; Fu, H.; et al. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef]
- Zhang, Q.; de Mejia, E.G. Protocatechuic acid attenuates adipogenesis-induced inflammation and mitochondrial dysfunction in 3T3-L1 adipocytes by regulation of AMPK pathway. J. Funct. Foods 2020, 69, 103972. [Google Scholar] [CrossRef]
- Kong, L.; Xu, M.; Qiu, Y.; Liao, M.; Zhang, Q.; Yang, L.; Zheng, G. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J. Food Biochem. 2021, 45, e13795. [Google Scholar] [CrossRef]
- Adam, Z.; Khamis, S.; Ismail, A.; Hamid, M. Ficus deltoidea: A potential alternative medicine for diabetes mellitus. Evid.-Based Complement. Altern. Med. 2012, 2012, 632763. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-induced TNFα and IL-6 signaling: The missing link between obesity and inflammation—Driven liver and colorectal cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Chen, X.; Gao, C.; Jiao, L.; Wang, J.; Xu, G.; Fu, H.; Guo, X.; Zhao, Y. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J. Bioenerg. Biomembr. 2011, 43, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Berger, J.; Hu, E.-D.; Szalkowski, D.; White-Carrington, S.; Spiegelman, B.M.; Moller, D.E. Negative regulation proliferator-activated gene expression of peroxisome receptor-y contributes to the antiadipogenic effects of tumor. Mol. Endocrinol. 1996, 10, 1457–1466. [Google Scholar] [PubMed]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.R.; Farmer, S.R. PPAR induces the insulin-dependent glucose transproter GLUT4 in the absence of C/EBP during the conversion of 3T3 fibroblast into adipocytes. Am. Soc. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP and PPAR controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Santiago, L.A.; Saguinsin, S.G.C.; Reyes, A.M.L.; Guerrero, R.P.; Nuguid, A.M.N.; Santos, A.C.N. Total phenolic and flavonoid contents and free radical scavenging components of Ficus nota Merr. (Moraceae) ethanolic leaf extract. Int. Food Res. J. 2017, 24, 2050–2058. [Google Scholar]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a naturally occurring phenolic compound inhibits LPS-induced iNOS and COX-2 expression in RAW264.7 cells by blocking NF-κB activation pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nworu, C.S.; Nwuke, H.C.; Akah, P.A.; Okoye, F.B.C.; Esimone, C.O. Extracts of Ficus exasperata leaf inhibit topical and systemic inflammation in rodents and suppress LPS-induced expression of mediators of inflammation in macrophages. J. Immunotoxicol. 2012, 10, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Son, H.Y.; Van Minh, C.; Kim, Y.H.; Park, Y. Methanol extract of Ficus leaf inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated microglia via the MAPK pathway. Phytother. Res. 2008, 22, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Swantek, J.L.; Cobb, M.H.; Geppert, T.D. Jun N-Terminal Kinase/Stress-Activated Protein Kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997, 17, 6274–6282. [Google Scholar] [CrossRef]
- Huang, F.; Cao, J.; Liu, Q.; Zou, Y.; Li, H.; Yin, T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1β induced in human endometriosis stromal cells in vitro. Int. J. Clin. Exp. Pathol. 2013, 6, 2129–2136. [Google Scholar]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Budluang, P.; Pitchakarn, P.; Ting, P.; Temviriyanukul, P.; Wongnoppawich, A.; Imsumran, A. Anti-inflammatory and anti-insulin resistance activities of aqueous extract from Anoectochilus burmannicus. Food Sci. Nutr. 2017, 5, 486–496. [Google Scholar] [CrossRef]
- Craig, A.P.; Fields, C.; Liang, N.; Kitts, D.; Erickson, A. Performance review of a fast HPLC-UV method for the quantification of chlorogenic acids in green coffee bean extracts. Talanta 2016, 154, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Chupeerach, C.; Temviriyanukul, P.; Thangsiri, S.; Inthachat, W.; Sahasakul, Y.; Aursalung, A.; Wongchang, P.; Sangkasa-Ad, P.; Wongpia, A.; Polpanit, A.; et al. Phenolic profiles and bioactivities of ten original lineage beans in thailand. Foods 2022, 11, 3905. [Google Scholar] [CrossRef] [PubMed]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of drying processes on phenolics and in vitro health-related activities of indigenous plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Chatthongpisut, R.; Schwartz, S.J.; Yongsawatdigul, J. Antioxidant activities and antiproliferative activity of Thai purple rice cooked by various methods on human colon cancer cells. Food Chem. 2015, 188, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Wang, Y.-K.; Yih, K.-H. DPPH free-radical scavenging ability, total phenolic content, and chemical composition analysis of forty-five kinds of essential oils. J. Cosmet. Sci. 2008, 59, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Bittersohl, H.; Steimer, W. Chapter 9—Intracellular concentrations of immunosuppressants. In Personalized Immunosuppression in Transplantation; Oellerich, M., Dasgupta, A., Eds.; Elsevier: San Diego, CA, USA, 2016; pp. 199–226. [Google Scholar]
- Buacheen, P.; Karinchai, J.; Inthachat, W.; Butkinaree, C.; Jankam, C.; Wongnoppavich, A.; Imsumran, A.; Chewonarin, T.; Pimpha, N.; Temviriyanukul, P.; et al. The Toxicological Assessment of Anoectochilus burmannicus Ethanolic-Extract-Synthesized Selenium Nanoparticles Using Cell Culture, Bacteria, and Drosophila melanogaster as Suitable Models. Nanomaterials 2023, 13, 2804. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: New York, NY, USA, 2015; pp. 333–348. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Suganthy, N.; Muniasamy, S.; Archunan, G. Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: In vitro and in vivo studies. Regul. Toxicol. Pharmacol. 2017, 92, 347–357. [Google Scholar] [CrossRef]




| FL Extracts | Total Phenolic (mg GAE/g Extract) | Total Flavonoid (mg CE/g Extract) | DPPH Assay SC50 1 (µg/mL) | ABTS Assay SC50 (µg/mL) |
|---|---|---|---|---|
| FLLE | 248.53 ± 1.46 ** | 55.78 ± 4.00 | 210.59 ± 6.13 | 33.59 ± 6.26 |
| FLRE | 208.31 ± 9.90 | 157.33 ± 1.36 *** | 83.12 ± 8.86 *** | 9.44 ± 3.26 ** |
| Chlorogenic Acid (CGAs) (mg/g Extract) | Apigenin (AP) (mg/g Extract) | |||||
|---|---|---|---|---|---|---|
| FL Extracts | Total Phenolic (mg CGAE/g Extract) | Total Flavonoid (mg APE/g Extract) | 3-CQA | 4-CQA | 5-CQA | Apigenin |
| FLLE | 517.28 ± 2.72 | 33.55 ± 1.20 | 39.85 ± 0.88 | 19.80 ± 0.52 | 26.00 ± 0.93 | ND 1 |
| FLRE | 111.96 ± 0.67 | 51.81 ± 2.88 | 4.98 ± 0.10 | 1.08 ± 0.06 | 0.46 ± 0.08 | 0.93 ± 0.09 |
| FLLE | FLRE | |
|---|---|---|
| Compounds | mg/g Extract | Mg/G Extract |
| Gallic acid | ND 1 | 0.08 |
| Chlorogenic acid (5-CQA) | 3.34 | 0.74 |
| 3,4-Dihydroxybenzoic acid | ND | 0.56 |
| Caffeic acid | 0.40 | 0.21 |
| Luteolin | ND | 0.24 |
| Genistein | ND | 1.40 |
| Apigenin | ND | 0.88 |
| Naringenin | ND | 0.53 |
| Extracts (µg/mL) | % Hemolysis (n = 5) |
|---|---|
| 0.05% Triton x-100 1 | 100 ± 0.00 |
| FLLE | |
| 200 | 4.55 ± 0.70 |
| 400 | 4.90 ± 0.79 |
| 600 | 5.41 ± 0.73 |
| 800 | 5.95 ± 0.65 |
| FLRE | |
| 200 | 5.59 ± 0.43 |
| 400 | 8.12 ± 1.36 |
| 600 | 10.70 ± 2.22 |
| 800 | 12.70 ± 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karinchai, J.; Buacheen, P.; Rodthayoy, D.; Yodweerapong, T.; Imsumran, A.; Wongnoppavich, A.; Chantarasuwan, B.; Suttisansanee, U.; Temviriyanukul, P.; Pitchakarn, P. Anti-Inflammatory and Anti-Adipocyte Dysfunction Effects of Ficus lindsayana Latex and Root Extracts. Pharmaceuticals 2024, 17, 287. https://doi.org/10.3390/ph17030287
Karinchai J, Buacheen P, Rodthayoy D, Yodweerapong T, Imsumran A, Wongnoppavich A, Chantarasuwan B, Suttisansanee U, Temviriyanukul P, Pitchakarn P. Anti-Inflammatory and Anti-Adipocyte Dysfunction Effects of Ficus lindsayana Latex and Root Extracts. Pharmaceuticals. 2024; 17(3):287. https://doi.org/10.3390/ph17030287
Chicago/Turabian StyleKarinchai, Jirarat, Pensiri Buacheen, Daraphan Rodthayoy, Tanpitcha Yodweerapong, Arisa Imsumran, Ariyaphong Wongnoppavich, Bhanumas Chantarasuwan, Uthaiwan Suttisansanee, Piya Temviriyanukul, and Pornsiri Pitchakarn. 2024. "Anti-Inflammatory and Anti-Adipocyte Dysfunction Effects of Ficus lindsayana Latex and Root Extracts" Pharmaceuticals 17, no. 3: 287. https://doi.org/10.3390/ph17030287