Putting a “C60 Ball” and Chain to Chlorin e6 Improves Its Cellular Uptake and Photodynamic Performances
Abstract
:1. Introduction
2. Results and Discussion
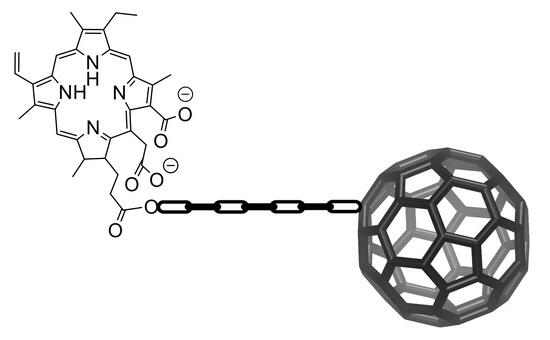
2.1. Synthesis and Characterization of the Ce6–C60 Dyad
2.2. Generation of ROS by the Ce6–C60 Dyad upon Red Light Irradiation
2.3. Cellular Uptake of the Ce6–C60 Dyad
2.4. Phototoxicity of the Ce6–C60 Dyad in A431 Cells Using Red Light Irradiation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fullerene Derivative and Ce6–C60 Dyad
3.3. Detection of ROS Produced upon Red Light Irradiation
3.4. Cellular Uptake
3.5. Cytotoxicity and Phototoxicity in A431 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J.; et al. Antitumor Effect of Photodynamic Therapy/Sonodynamic Therapy/Sono-Photodynamic Therapy of Chlorin e6 and Other Applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Juzienne, A. Chlorin E6-Based Photosensitizers for Photodynamic Therapy and Photodiagnosis. Photodiagnosis Photodyn. Ther. 2009, 6, 94–96. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes in Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic Therapy with Fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as Photosensitizers in Photodynamic Therapy: Pros and Cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef]
- Hou, W.; Shi, G.; Wu, S.; Mo, J.; Shen, L.; Zhang, X.; Zhu, Y. Application of Fullerenes as Photosensitizers for Antimicrobial Photodynamic Inactivation: A Review. Front. Microbiol. 2022, 13, 957698. [Google Scholar] [CrossRef]
- Cantelli, A.; Malferrari, M.; Mattioli, E.J.; Marconi, A.; Mirra, G.; Soldà, A.; Marforio, T.D.; Zerbetto, F.; Rapino, S.; Di Giosia, M.; et al. Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation. Nanomaterials 2022, 12, 3501. [Google Scholar] [CrossRef]
- Di Giosia, M.; Nicolini, F.; Ferrazzano, L.; Soldà, A.; Valle, F.; Cantelli, A.; Marforio, T.D.; Bottoni, A.; Zerbetto, F.; Montalti, M.; et al. Stable and Biocompatible Monodispersion of C60 in Water by Peptides. Bioconjugate Chem. 2019, 30, 808–814. [Google Scholar] [CrossRef]
- Di Giosia, M.; Soldà, A.; Seeger, M.; Cantelli, A.; Arnesano, F.; Nardella, M.I.; Mangini, V.; Valle, F.; Montalti, M.; Zerbetto, F.; et al. A Bio-Conjugated Fullerene as a Subcellular-Targeted and Multifaceted Phototheranostic Agent. Adv. Funct. Mater. 2021, 31, 2101527. [Google Scholar] [CrossRef]
- Serda, M.; Szewczyk, G.; Krzysztyńska-Kuleta, O.; Korzuch, J.; Dulski, M.; Musioł, R.; Sarna, T. Developing [60]Fullerene Nanomaterials for Better Photodynamic Treatment of Non-Melanoma Skin Cancers. ACS Biomater. Sci. Eng. 2020, 6, 5930–5940. [Google Scholar] [CrossRef] [PubMed]
- Serda, M.; Ware, M.J.; Newton, J.M.; Sachdeva, S.; Krzykawska-Serda, M.; Nguyen, L.; Law, J.; Anderson, A.O.; Curley, S.A.; Wilson, L.J.; et al. Development of Photoactive Sweet-C 60 for Pancreatic Cancer Stellate Cell Therapy. Nanomedicine 2018, 13, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Antoku, D.; Sugikawa, K.; Ikeda, A. Photodynamic Activity of Fullerene Derivatives Solubilized in Water by Natural-Product-Based Solubilizing Agents. Chem. A Eur. J. 2019, 25, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.d.G.H.; Smith, K.M. Amino Acid Derivatives of Chlorin-E6—A Review. Molecules 2023, 28, 3479. [Google Scholar] [CrossRef]
- Osaki, T.; Hibino, S.; Yokoe, I.; Yamaguchi, H.; Nomoto, A.; Yano, S.; Mikata, Y.; Tanaka, M.; Kataoka, H.; Okamoto, Y. A Basic Study of Photodynamic Therapy with Glucose-Conjugated Chlorin E6 Using Mammary Carcinoma Xenografts. Cancers 2019, 11, 63. [Google Scholar] [CrossRef]
- Shinoda, Y.; Kujirai, K.; Aoki, K.; Morita, M.; Masuda, M.; Zhang, L.; Kaixin, Z.; Nomoto, A.; Takahashi, T.; Tsuneoka, Y.; et al. Novel Photosensitizer β-Mannose-Conjugated Chlorin E6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells. Pharmaceuticals 2020, 13, 316. [Google Scholar] [CrossRef]
- Thapa Magar, T.B.; Lee, J.; Lee, J.H.; Jeon, J.; Gurung, P.; Lim, J.; Kim, Y.-W. Novel Chlorin E6-Curcumin Derivatives as a Potential Photosensitizer: Synthesis, Characterization, and Anticancer Activity. Pharmaceutics 2023, 15, 1577. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, M.; Hu, X.; Du, Q.; Zhao, Z.; Jiang, Y.; Luan, Y. Nanoengineering of a Newly Designed Chlorin E6 Derivative for Amplified Photodynamic Therapy: Via Regulating Lactate Metabolism. Nanoscale 2021, 13, 11953–11962. [Google Scholar] [CrossRef]
- Dias, L.D.; Mfouo-Tynga, I.S. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics 2020, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.; Mattioli, E.J.; Ingargiola, F.; Giugliano, G.; Marforio, D.; Prodi, L.; Di Giosia, M.; Calvaresi, M. Dissecting the Interactions between Chlorin E6 and Human Serum Albumin. Molecules 2023, 28, 2348. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kang, J.K.; Won, W.R.; Park, J.Y.; Han, S.M.; Le, T.N.; Kim, J.C.; Her, J.; Shin, Y.; Oh, K.T. Co-Delivery of D-(KLAKLAK)2 Peptide and Chlorin E6 Using a Liposomal Complex for Synergistic Cancer Therapy. Pharmaceutics 2019, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Yoo, J.; Lee, D.; Jang, S.Y.; Kwon, S.; Koo, H. Chlorin E6-Loaded PEG-PCL Nanoemulsion for Photodynamic Therapy and in vivo Drug Delivery. Int. J. Mol. Sci. 2019, 20, 3958. [Google Scholar] [CrossRef]
- Peng, P.C.; Hong, R.L.; Tsai, T.; Chen, C.T. Co-Encapsulation of Chlorin E6 and Chemotherapeutic Drugs in a Pegylated Liposome Enhance the Efficacy of Tumor Treatment: Pharmacokinetics and Therapeutic Efficacy. Pharmaceutics 2019, 11, 617. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin E6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef]
- Nasr, S.; Rady, M.; Sebak, A.; Gomaa, I.; Fayad, W.; El Gaafary, M.; Abdel-kader, M.; Syrovets, T.; Simmet, T. A Naturally Derived Carrier for Photodynamic Treatment of Squamous Cell Carcinoma: In Vitro and in Vivo Models. Pharmaceutics 2020, 12, 494. [Google Scholar] [CrossRef]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C60 as a Multifunctional System for Drug and Gene Delivery. Nanoscale 2011, 3, 4035–4041. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical Engineers Get to Revisit an Old Friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-Based Delivery Systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Antoku, D.; Satake, S.; Mae, T.; Sugikawa, K.; Funabashi, H.; Kuroda, A.; Ikeda, A. Improvement of Photodynamic Activity of Lipid–Membrane-Incorporated Fullerene Derivative by Combination with a Photo-Antenna Molecule. Chem. A Eur. J. 2018, 24, 7335–7339. [Google Scholar] [CrossRef] [PubMed]
- Shimada, R.; Hino, S.; Yamana, K.; Kawasaki, R.; Konishi, T.; Ikeda, A. Improvement of Photodynamic Activity by a Stable System Consisting of a C60 Derivative and Photoantenna in Liposomes. ACS Med. Chem. Lett. 2022, 13, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Antoku, D.; Ohdake, R.; Sugikawa, K.; Ikeda, A. Bacterial Elimination via the Photodynamic Activity of a Fullerene/Light-Harvesting Antenna Molecule Assembled System Integrated into Liposome Membranes. Nanoscale Adv. 2020, 2, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Lopez, E.J.; Sarotti, A.M.; Martínez, S.R.; Macor, L.P.; Durantini, J.E.; Renfige, M.; Gervaldo, M.A.; Otero, L.A.; Durantini, A.M.; Durantini, E.N.; et al. BOPHY-Fullerene C60 Dyad as a Photosensitizer for Antimicrobial Photodynamic Therapy. Chem. A Eur. J. 2022, 28, e202103884. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Ge, J.; Wu, J.; Zhang, G.; Chen, D.; Zhang, W.; Zhang, Y.; Zou, T.; Zhen, M.; Wang, C.; et al. Fullerene/Photosensitizer Nanovesicles as Highly Efficient and Clearable Phototheranostics with Enhanced Tumor Accumulation for Cancer Therapy. Biomaterials 2016, 103, 75–85. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Almodovar, V.A.S.; Gsponer, N.S.; Bertolotti, S.; Tomé, A.C.; Durantini, E.N. Diketopyrrolopyrrole-Fullerene C60 Architectures as Highly Efficient Heavy Atom-Free Photosensitizers: Synthesis, Photophysical Properties and Photodynamic Activity. Org. Biomol. Chem. 2020, 18, 1449–1461. [Google Scholar] [CrossRef]
- Rybkin, A.Y.; Belik, A.Y.; Goryachev, N.S.; Mikhaylov, P.A.; Kraevaya, O.A.; Filatova, N.V.; Parkhomenko, I.I.; Peregudov, A.S.; Terent’ev, A.A.; Larkina, E.A.; et al. Self-Assembling Nanostructures of Water-Soluble Fullerene [60]–Chlorin E6 Dyads: Synthesis, Photophysical Properties, and Photodynamic Activity. Dyes Pigments 2020, 180, 108411. [Google Scholar] [CrossRef]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Enhancing Type I Photochemistry in Photodynamic Therapy under Near Infrared Light by Using Antennae–Fullerene Complexes. Cytom. Part A 2018, 93, 997–1003. [Google Scholar] [CrossRef]
- Bertran, J.C.; Montforts, F.P. Synthesis of a Chlorin Fullerene Dyad for Artificial Photosynthesis. Eur. J. Org. Chem. 2017, 12, 1608–1617. [Google Scholar] [CrossRef]
- Di Giosia, M.; Genovese, D.; Cantelli, A.; Cingolani, M.; Rampazzo, E.; Strever, G.; Tavoni, M.; Zaccheroni, N.; Calvaresi, M.; Prodi, L. Synthesis and Characterization of a Reconstituted Myoglobin-Chlorin E6 Adduct for Theranostic Applications. J. Porphyr. Phthalocyanines 2020, 24, 887–893. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef] [PubMed]
- Parkhats, M.V.; Galievsky, V.A.; Stashevsky, A.S.; Trukhacheva, T.V.; Dzhagarov, B.M. Dynamics and Efficiency of the Photosensitized Singlet Oxygen Formation by Chlorin E6: The Effects of the Solution PH and Polyvinylpyrrolidone. Opt. Spectrosc. 2009, 107, 974–980. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–662649. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; He, P.; Wang, Z.; Tang, B.Z. A Feasible Strategy of Fabricating Type i Photosensitizer for Photodynamic Therapy in Cancer Cells and Pathogens. ACS Nano 2021, 15, 7735–7743. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Pawlak, A.; Satti, M.; Lee, H.; Wharton, T.; Gali, H.; Sarna, T.; Hamblin, M.R. Functionalized Fullerenes Mediate Photodynamic Killing of Cancer Cells: Type I versus Type II Photochemical Mechanism. Free Radic. Biol. Med. 2007, 43, 711–719. [Google Scholar] [CrossRef]
- Gradova, M.A.; Movchan, T.G.; Khudyaeva, I.S.; Chernyad’ev, A.Y.; Plotnikova, E.V.; Lobanov, A.V.; Belykh, D.V. Synthesis of the Novel Cationic Chlorin Derivatives with a Phytol Fragment on the Periphery of the Macrocycle and Their Aggregation State in Aqueous Surfactant Solutions. Macroheterocycles 2020, 13, 23–32. [Google Scholar] [CrossRef]
- Gradova, M.A.; Gradov, O.V.; Lobanov, A.V.; Bychkova, A.V.; Nikolskaya, E.D.; Yabbarov, N.G.; Mollaeva, M.R.; Egorov, A.E.; Kostyukov, A.A.; Kuzmin, V.A.; et al. Characterization of a Novel Amphiphilic Cationic Chlorin Photosensitizer for Photodynamic Applications. Int. J. Mol. Sci. 2023, 24, 345. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive Cationic Functionalization of Chlorin Derivatives for Antimicrobial Photodynamic Inactivation and Related Vancomycin Conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Pylina, Y.I.; Khudyaeva, I.S.; Startseva, O.M.; Shadrin, D.M.; Shevchenko, O.G.; Velegzhaninov, I.O.; Kukushkina, N.V.; Berezin, D.B.; Belykh, D.V. Dark and Photoinduced Cytotoxicity of Cationic Chlorin E6 Derivatives with Different Numbers of Charged Groups. Macroheterocycles 2021, 14, 317–322. [Google Scholar] [CrossRef]
- Kruijtzer, J.A.W.; Hofmeyer, L.J.F.; Heerma, W.; Versluis, C.; Liskamp, R.M.J. Solid-Phase Syntheses of Peptoids Using Fmoc-Protected N-Substituted Glycines: The Synthesis of (Retro)Peptoids of Leu-Enkephalin and Substance P. Chem. A Eur. J. 1998, 4, 1570–1580. [Google Scholar] [CrossRef]
- Kordatos, K.; Da Ros, T.; Bosi, S.; Vázquez, E.; Bergamin, M.; Cusan, C.; Pellarini, F.; Tomberli, V.; Baiti, B.; Pantarotto, D.; et al. Novel Versatile Fullerene Synthons. J. Org. Chem. 2001, 66, 4915–4920. [Google Scholar] [CrossRef] [PubMed]
- Ulfo, L.; Cantelli, A.; Petrosino, A.; Costantini, P.E.; Nigro, M.; Starinieri, F.; Turrini, E.; Zadran, S.K.; Zuccheri, G.; Saporetti, R.; et al. Orthogonal Nanoarchitectonics of M13 Phage for Receptor Targeted Anticancer Photodynamic Therapy. Nanoscale 2022, 14, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Cantelli, A.; Malferrari, M.; Soldà, A.; Simonetti, G.; Forni, S.; Toscanella, E.; Mattioli, E.J.; Zerbetto, F.; Zanelli, A.; Di Giosia, M.; et al. Human Serum Albumin–Oligothiophene Bioconjugate: A Phototheranostic Platform for Localized Killing of Cancer Cells by Precise Light Activation. JACS Au 2021, 1, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Bortot, B.; Apollonio, M.; Baj, G.; Andolfi, L.; Zupin, L.; Crovella, S.; Di Giosia, M.; Cantelli, A.; Saporetti, R.; Ulfo, L.; et al. Advanced Photodynamic Therapy with an Engineered M13 Phage Targeting EGFR: Mitochondrial Localization and Autophagy Induction in Ovarian Cancer Cell Lines. Free Radic. Biol. Med. 2022, 179, 242–251. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sante, M.; Kaltenbrunner, A.; Lombardo, M.; Danielli, A.; Costantini, P.E.; Di Giosia, M.; Calvaresi, M. Putting a “C60 Ball” and Chain to Chlorin e6 Improves Its Cellular Uptake and Photodynamic Performances. Pharmaceuticals 2023, 16, 1329. https://doi.org/10.3390/ph16091329
Di Sante M, Kaltenbrunner A, Lombardo M, Danielli A, Costantini PE, Di Giosia M, Calvaresi M. Putting a “C60 Ball” and Chain to Chlorin e6 Improves Its Cellular Uptake and Photodynamic Performances. Pharmaceuticals. 2023; 16(9):1329. https://doi.org/10.3390/ph16091329
Chicago/Turabian StyleDi Sante, Manuele, Alena Kaltenbrunner, Marco Lombardo, Alberto Danielli, Paolo Emidio Costantini, Matteo Di Giosia, and Matteo Calvaresi. 2023. "Putting a “C60 Ball” and Chain to Chlorin e6 Improves Its Cellular Uptake and Photodynamic Performances" Pharmaceuticals 16, no. 9: 1329. https://doi.org/10.3390/ph16091329