Application of tris-(4,7-Diphenyl-1,10 phenanthroline)ruthenium(II) Dichloride to Detection of Microorganisms in Pharmaceutical Products †
Abstract
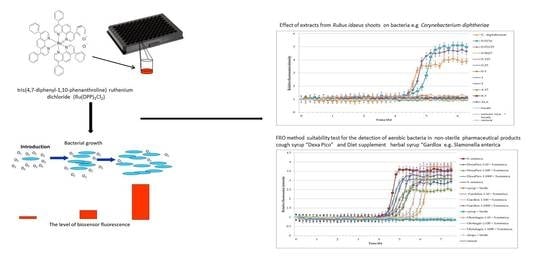
:1. Introduction
2. Results and Discussion
2.1. Effect of Dimethyl Sulfoxide against Bacteria
2.2. Effect of Plant Extracts against Bacteria
2.3. FOR Method Suitability Test for the Detection of Aerobic Bacteria in Non-Sterile Pharmaceutical Products
2.4. FOR Method Suitability Test for the Detection of Aerobic Bacteria in Sterile Pharmaceutical Products
3. Materials and Methods
3.1. Materials
3.2. Microorganisms Strains and Growth Conditions
3.3. Synthesis of tris-(4,7-Diphenyl-1,10-phenanthroline)ruthenium(II) Chloride (Ru(DPP)3Cl2) Biosensor and Coating the Walls of the 96-Well Microtiter Plates
3.4. Determination the Antimicrobial Properties of Compounds Using the Microbroth Dilution Method
3.5. Measurement of the Effect of Selected Chemicals on Bacteria by Fluorescence Optical Respirometry
3.6. FOR Method Suitability Test for the Detection of Aerobic Bacteria in Sterile and Non-Sterile Pharmaceutical Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Pharmacopoeia, 10th ed.; European Commision: Luxembourg, 2020.
- Hobson, N.S.; Tothill, I.; Tuner, A.P.F. Microbial detection. Biosens. Bioelectron. 1996, 11, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G.M. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef] [PubMed]
- Meighen, E.A. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 1991, 55, 123–142. [Google Scholar] [CrossRef]
- Farris, L.; Habteselassie, M.Y.; Perry, L.; Chen, Y.; Turco, R.; Reuhs, B.; Applegate, B. Luminescence techniques for the detection of bacterial pathogens. In Priciples of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer Sciences + Business Media: New York, NY, USA, 2008; Part II, Chapter 10; pp. 214–230. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentration. J. Antim. Chemoth. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felmingham, D.; Brown, D.F.J. Instrumentation in antimicrobial susceptibility testing. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 81–85. [Google Scholar] [CrossRef] [Green Version]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar] [CrossRef] [Green Version]
- Idelevich, E.A.; Becker, K. How to accelerate antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2019, 25, 1347–1355. [Google Scholar] [CrossRef]
- Olsen, E.; Vainrub, A.; Vodyanoy, V. Acoustic wave (TCM) biosensors: Weighing bacteria. In Priciples of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer Sciences + Business Media: New York, NY, USA, 2008; Part II, Chapter 12; pp. 255–298. [Google Scholar]
- Deng, Y.; Beahm, D.R.; Ionov, S.; Measuring, R.S. Measuring and modeling energy and power consumption in living microbial cells with a synthetic ATP reporter. BMC Biol. 2021, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.M. Manual and automated instrumentation for identyfication of Enterobacteriaceae and other aerobic gram-negative bacilli. Clin. Microbiol. Rev. 2005, 18, 147–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, S.; Ardito, F.; Sechi, L.; Sanguinetti, M.; Molicotti, P.; Delogu, G.; Pinna, M.P.; Nacci, A.; Fadda, G. Evaluation of nonradiometric system (Bactec900MB) for detection of Mycobacteria in human clinical samples. J. Clin. Microbiol. 1997, 35, 2072–2075. [Google Scholar] [CrossRef] [Green Version]
- Huynh, G.T.; Kesarwani, V.; Walker, J.A.; Frith, J.E.; Meagher, L.; Corrie, S.R. Review: Nanomaterials for Reactive Oxygen Species Detection and Monitoring in Biological Environments. Front. Chem. 2021, 9, 728717. [Google Scholar] [CrossRef] [PubMed]
- Papkovsky, D.B.; Kerry, J.P. Oxygen Sensor-Based Respirometry and the Landscape of Microbial Testing Methods as Applicable to Food and Beverage Matrices. Sensors 2023, 23, 4519. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, R.; Turecka, K.; Orlewska, C.; Werel, W. Comparison of fluorescence optical respirometry and microbroth dilution methods for testing antimicrobial compounds. J. Microbiol. Methods 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Jasionek, G.; Ogurtsov, V.; Papkovsky, D. Rapid detection and respirometric profiling of aerobic bacteria on panels of selective media. J. Appl. Microb. 2012, 114, 423–432. [Google Scholar] [CrossRef]
- Turecka, K.; Chylewska, A.; Dąbrowska, A.M.; Hałasa, R.; Orlewska, C.; Waleron, K. Ru(II)Oxygen Sensors for Co(III)Complexes and Amphotericin B Antifungal Activity Detection by Phosphorescence Optical Respirometry. Int. J. Mol. Sci. 2023, 24, 8744. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-Based Oxygen (O2) Sensors for On-Chip Monitoring of Cell, Tissue and Organ Metabolism. Biosensors 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Elisseeva, S.; Bukulin, A.; Kerry, J.P.; Papkovsky, D.P. Facile biosensor-based system for on-site quantification of total viable counts in food and environmental swabs. Biosens. Bioelectron. 2021, 176, 112938. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Elisseeva, S.; Smyth, C.; Cruz-Romero, M.; Kerry, J.P.; Duffy, G.; Papkovsky, D.B. A sensor-based system for rapid on-site testing of microbial contamination in meat samples and carcasses. J Appl Microbiol. 2022, 132, 1210–1220. [Google Scholar] [CrossRef]
- Santovito, E.; Elisseeva, S.; Cruz-Romero, M.C.; Duffy, G.; Kerry, J.P.; Papkovsky, D.B. A Simple Sensor System for Onsite Monitoring of O2 in Vacuum-Packed Meats during the Shelf Life. Sensors 2021, 21, 4256. [Google Scholar] [CrossRef]
- Pomarnacka, E.; Bednarski, P.J.; Reszka, P.; Dziemidowicz-Borys, E.; Bieńczak, A.; Werel, W.; Hałasa, R. Synthesis and biological activity of new 2-amino-8-chloro-5,5-dioxo[1,2,4]triazolo[2,3-b][1,4,2]benzodithiazines. Eur. J. Med. Chem. 2006, 41, 633–639. [Google Scholar] [CrossRef]
- Sączewski, F.; Kornicka, A.; Gdaniec, M.; Hałasa, R.; Werel, W. First stable 0-amidinylhydroxylamines and their transformations into sulfenamides by intramolecular 1,5-O S amine migration. Eur. J. Org. Chem. 2004, 2004, 3511–3516. [Google Scholar] [CrossRef]
- Sączewski, J.; Gdaniec, M. First tandem nucleophilic addition–electrophilic amination reaction of Eschenmoser’s salt: Synthesis of ciclic and spiro-fused hydrazonium salt. Tetrahedron Lett. 2007, 48, 7624–7627. [Google Scholar] [CrossRef]
- Sączewski, F.; Stencel, A.; Bieńczak, A.M.; Langowska, K.A.; Michaelis, M.; Werel, W.; Hałasa, R.; Reszka, P. Structure-activity relationships of novel heteroaryl-acrylonitriles as cytotoxic and antibacterial agents. Eur. J. Med. Chem. 2008, 43, 1847–1857. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Balicki, I.; Szadkowski, M.; Balicka, A.; Zwolska, J. Clinical study on the application of dexamethasone and cyclosporine/dimethyl sulfoxide combination eye drops in the initial therapy of chronic superficial keratitis in dogs. Pol. J. Vet. Sci. 2021, 24, 415–423. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.C.; Papkovsky, D.B. Rapid high-throughput assessment of aerobic bacteria in complex samples by fluorescence-6 based oxygen respirometry. Appl. Environ. Microbiol. 2006, 72, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Violante, I.M.P.; Hamerski, L.; Garcez, W.S.; Batista, A.L.; Rodrigues Chang, M.; Pott, V.J.; Rodrigues Garcez, F. Antimicrobial activity of some medicinal plants from the cerrado of the central-western region of Brazil. Braz. J. Microbiol. 2012, 43, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Justyna Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R.J. Biological activities of leaf extracts from selected Kalanchoe species and their relationship with bufadienolides content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef]
- Kula, M.; Majdan, M.; Radwańska, A.; Nasal, A.; Hałasa, R.; Głód, D.; Matkowski, A.; Krauze-Baranowska, M. Chemical composition and biological activity of the fruits from Lonicera caerulea var. edulis ‘Wojtek’. Acad. J. Med. Plants 2013, 1, 141–148. [Google Scholar]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Watts, R.J.; Crosby, G.A. Spectroscopic characterization of complexes od ruthenium(II) and iridium(III) with 4,4′-diphenyl-2,-2’bipyridine and 4,7-diphenyl-1,10 phenanthroline. J. Am. Chem. Soc. 1971, 93, 3184–3188. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hałasa, R.; Turecka, K.; Smoktunowicz, M.; Mizerska, U.; Orlewska, C. Application of tris-(4,7-Diphenyl-1,10 phenanthroline)ruthenium(II) Dichloride to Detection of Microorganisms in Pharmaceutical Products. Pharmaceuticals 2023, 16, 856. https://doi.org/10.3390/ph16060856
Hałasa R, Turecka K, Smoktunowicz M, Mizerska U, Orlewska C. Application of tris-(4,7-Diphenyl-1,10 phenanthroline)ruthenium(II) Dichloride to Detection of Microorganisms in Pharmaceutical Products. Pharmaceuticals. 2023; 16(6):856. https://doi.org/10.3390/ph16060856
Chicago/Turabian StyleHałasa, Rafał, Katarzyna Turecka, Magdalena Smoktunowicz, Urszula Mizerska, and Czesława Orlewska. 2023. "Application of tris-(4,7-Diphenyl-1,10 phenanthroline)ruthenium(II) Dichloride to Detection of Microorganisms in Pharmaceutical Products" Pharmaceuticals 16, no. 6: 856. https://doi.org/10.3390/ph16060856