Immunohistochemical Expression of Glutathione Peroxidase 1 (Gpx-1) as an Independent Prognostic Factor in Colon Adenocarcinoma Patients
Abstract
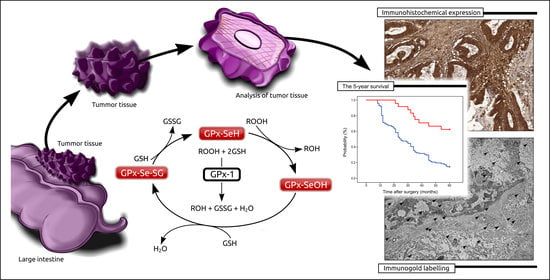
:1. Introduction
2. Results
2.1. Characteristics of Patients Included in the Study
2.2. The Associations between the Immunohistochemical Expression of Gpx-1 and Patients’ Clinical Features
2.3. The Prognostic Significance of Gpx-1 Expression in Relation to 5-Year Survival
2.4. Detection of Gpx-1 at the Cellular Level by the Use of TEM
2.5. Analysis of Gpx-1 in Selected Cancer Cell Lines by the Use of the Western Blot Method
3. Discussion
4. Materials and Methods
4.1. Patients and Tumour Samples
4.2. Immunohistochemical Staining
4.3. Statistical Analysis
4.4. Immunogold Electron Microscopy
4.5. Cell Lines
4.6. Western Blot
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.L.; Gilreath, K.; Keyes, D. Colorectal cancer disparities beyond biology Screening treatment access. Front. Biosci. 2017, 22, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Harbiyeli, I.F.C.; Burtea, D.E.; Ivan, E.T.; Streață, I.; Nicoli, E.R.; Uscatu, D.; Șerbănescu, M.-S.; Ioana, M.; Vilmann, P.; Săftoiu, A. Assessing Putative Markers of Colorectal Cancer Stem Cells: From Colonoscopy to Gene Expression Profiling. Diagnostics 2022, 12, 2280. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Vabi, B.W.; Gibbs, J.F.; Parker, G.S. Implications of the growing incidence of global colorectal cancer. J. Gastrointest. Oncol. 2021, 12 (Suppl. 2), S387–S398. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Schwarz, M.; Löser, A.; Cheng, Q.; Wichmann-Costaganna, M.; Schädel, P.; Werz, O.; Arnér, E.S.; Kipp, A.P. Side-by-side comparison of recombinant human glutathione peroxidases identifies overlapping substrate specificities for soluble hydroperoxides. Redox Biol. 2023, 59, 102593. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef]
- Koeberle, S.C.; Gollowitzer, A.; Laoukili, J.; Kranenburg, O.; Werz, O.; Koeberle, A.; Kipp, A.P. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms. Redox Biol. 2020, 28, 101388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Flores, J.N.; Shetty, S.P.; Dubey, A.; Copeland, P. The molecular biology of selenocysteine. Biomol. Concepts 2013, 4, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, G.-W.; Wang, N.; Wang, Y.-J. GPX1 Pro198Leu polymorphism and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010, 124, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bănescu, C.; Trifa, A.P.; Voidăzan, S.; Moldovan, V.G.; Macarie, I.; Lazar, E.B.; Dima, D.; Duicu, C.; Dobreanu, M. CAT, GPX1, MnSOD, GSTM1, GSTT1, and GSTP1 Genetic Polymorphisms in Chronic Myeloid Leukemia: A Case-Control Study. Oxidative Med. Cell. Longev. 2014, 2014, 875861. [Google Scholar] [CrossRef]
- Hansen, R.; Sæbø, M.; Skjelbred, C.F.; Nexø, B.A.; Hagen, P.C.; Bock, G.; Lothe, I.M.B.; Johnson, E.; Aase, S.; Hansteen, I.-L.; et al. GPX Pro198Leu and OGG1 Ser326Cys polymorphisms and risk of development of colorectal adenomas and colorectal cancer. Cancer Lett. 2005, 229, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.D.; Krath, B.N.; Frederiksen, K.; Tjønneland, A.; Overvad, K.; Roswall, N.; Loft, S.; Dragsted, L.O.; Vogel, U.; Raaschou-Nielsen, O. GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, interaction with alcohol consumption and smoking, and risk of colorectal cancer. Mutat. Res. Mol. Mech. Mutagen. 2009, 664, 13–19. [Google Scholar] [CrossRef]
- Al-Sheneber, I.F.; Shibata, H.R.; Sampalis, J.; Jothy, S. Prognostic significance of proliferating cell nuclear antigen expression in colorectal cancer. Cancer 1993, 71, 1954–1959. [Google Scholar]
- Lavezzi, A.M.; Ottaviani, G.; De Ruberto, F.; Fichera, G.; Matturri, L. Prognostic significance of different biomarkers (DNA content, PCNA, karyotype) in colorectal adenomas. Anticancer. Res. 2002, 22, 2077–2081. [Google Scholar]
- Diamond, A.M. The Subcellular Location of Selenoproteins and the Impact on Their Function. Nutrients 2015, 7, 3938–3948. [Google Scholar] [CrossRef]
- Wei, R.; Qiu, H.; Xu, J.; Mo, J.; Liu, Y.; Gui, Y.; Huang, G.; Zhang, S.; Yao, H.; Huang, X.; et al. Expression and prognostic potential of GPX1 in human cancers based on data mining. Ann. Transl. Med. 2020, 8, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Y.; He, Y.; Xiao, Y.; Wang, Q.; Zhao, Y.; Zhang, T.; Wu, C.; Xie, Y.; Zhou, J.; et al. GPX1-associated prognostic signature predicts poor survival in patients with acute myeloid leukemia and involves in immunosuppression. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, T.; Li, S.; Ruan, H. GPX1, a biomarker for the diagnosis and prognosis of kidney cancer, promotes the progression of kidney cancer. Aging 2019, 11, 12165–12176. [Google Scholar] [CrossRef]
- Lee, J.R.; Roh, J.-L.; Lee, S.M.; Park, Y.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Overexpression of glutathione peroxidase 1 predicts poor prognosis in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, H.; You, Y.; Zhang, J.; Chen, R. High Gpx1 expression predicts poor survival in laryngeal squamous cell carcinoma. Auris Nasus Larynx 2018, 45, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.F.; Eldeen, M.A.; Khalifa, A.S.; Elsawy, W.H.; Eed, E.M.; El Askary, A.; Eid, R.A.; Soltan, M.A.; Raafat, N. Assessment of Glutathione Peroxidase-1 (GPX1) Gene Expression as a Specific Diagnostic and Prognostic Biomarker in Malignant Pleural Mesothelioma. Diagnostics 2021, 11, 2285. [Google Scholar] [CrossRef]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Hua, J.; Zhang, B.; Xu, J.; Yu, X. Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3β/Snail signaling. Oncogene 2018, 37, 5843–5857. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, J.; Liang, C.; Liu, J.; Hua, J.; Zhang, Y.; Ni, Q.; Shi, S.; Yu, X. GPx1 is involved in the induction of protective autophagy in pancreatic cancer cells in response to glucose deprivation. Cell Death Dis. 2018, 9, 1187. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Vorschlag zur einheitlichen Definition eines Immunreaktiven Score (IRS) für den immunhisto-chemischen Ostrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Der Pathologe 1987, 8, 138–140. [Google Scholar]
- Alnuaimi, A.R.; Bottner, J.; Nair, V.A.; Ali, N.; Alnakhli, R.; Dreyer, E.; Talaat, I.M.; Busch, H.; Perner, S.; Kirfel, J.; et al. Immunohistochemical Expression Analysis of Caldesmon Isoforms in Colorectal Carcinoma Reveals Interesting Correlations with Tumor Characteristics. Int. J. Mol. Sci. 2023, 24, 2275. [Google Scholar] [CrossRef]






| N (Number of Cases) | % | ||
|---|---|---|---|
| Gender | Females | 71 | 49.65 |
| Males | 72 | 50.35 | |
| Age (years) | ≤60 years | 53 | 37.06 |
| 61–75 years | 50 | 34.97 | |
| >75 years | 40 | 27.97 | |
| M ± SD | 65.03 ± 12.72 | ||
| Me (Q1–Q3) | 65 (56–77) | ||
| Min–max | 33–89 | ||
| Grade of histological differentiation (G) | G1 | 34 | 23.78 |
| G2 | 68 | 47.55 | |
| G3 | 41 | 28.67 | |
| Depth of invasion (T) | T1 | 23 | 16.08 |
| T2 | 21 | 14.69 | |
| T3 | 74 | 51.75 | |
| T4 | 25 | 17.48 | |
| Regional lymph node involvement | N0 | 67 | 46.85 |
| N1 | 39 | 27.27 | |
| N2 | 37 | 25.88 | |
| Location of tumour | Right-sided tumours | 75 | 52.45 |
| Left-sided tumours | 68 | 47.55 | |
| Angioinvasion | No | 39 | 27.27 |
| Yes | 104 | 72.73 | |
| Immunohistochemical expression of PCNA | Low | 65 | 45.45 |
| High | 78 | 54.55 | |
| Staging | I | 34 | 23.78 |
| II | 32 | 22.38 | |
| III | 77 | 53.84 | |
| The Immunoexpression Level of Gpx-1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| PCNA expression | Low | 55 | (38.46%) | 10 | (6.99%) | p = 0.463 |
| High | 14 | (9.79%) | 64 | (44.76%) | p = 0.540 | |
| The Immunoexpression Level of Gpx-1 | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Age (years) | ≤60 years | 29 | (54.72%) | 24 | (45.28%) | p = 0.317 |
| 61–75 years | 20 | (40.00%) | 30 | (60.00%) | ||
| >75 years | 20 | (50.00%) | 20 | (50.00%) | ||
| Gender | Females | 37 | (52.11%) | 34 | (47.89%) | p = 0.359 |
| Males | 32 | (44.44%) | 40 | (55.56%) | ||
| Grade of histological differentiation (G) | G1 | 30 | (88.24%) | 4 | (11.76%) | p < 0.001 |
| G2 | 33 | (48.53%) | 35 | (51.47%) | ||
| G3 | 6 | (14.63%) | 35 | (85.37%) | ||
| Depth of invasion (T) | T1 | 20 | (86.96%) | 3 | (13.04%) | p < 0.001 |
| T2 | 11 | (52.38%) | 10 | (47.62%) | ||
| T3 | 30 | (40.54%) | 44 | (59.46%) | ||
| T4 | 8 | (32.00%) | 17 | (68.00%) | ||
| Regional lymph node involvement | N0 | 30 | (44.78%) | 37 | (55.22%) | p = 0.737 |
| N1 | 20 | (51.28%) | 19 | (48.72%) | ||
| N2 | 19 | (51.35%) | 18 | (48.65%) | ||
| Localisation | Left-sided tumours | 39 | (52.00%) | 36 | (48.00%) | p = 0.346 |
| Right-sided tumours | 30 | (44.12%) | 38 | (55.88%) | ||
| Angioinvasion | Yes | 34 | (87.18%) | 5 | (12.82%) | p < 0.001 |
| No | 35 | (33.65%) | 69 | (66.35%) | ||
| PCNA expression | Low | 55 | (84.62%) | 10 | (15.38%) | p < 0.001 |
| High | 14 | (17.95%) | 64 | (82.05%) | ||
| Staging | I | 21 | (61.76%) | 13 | (38.24%) | p = 0.020 |
| II | 9 | (28.13%) | 23 | (71.88%) | ||
| III | 39 | (50.65%) | 38 | (49.35%) | ||
| Prognostic Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender | 1.043 | 0.704–1.543 | 0.835 | – | – | – |
| Age | 1.008 | 0.993–1.023 | 0.316 | – | – | – |
| Staging | 1.370 | 1.072–1.752 | 0.012 | 0.971 | 0.573–1.645 | 0.913 |
| Grade of histological differentiation | 2.887 | 2.151–3.876 | <0.001 | 1.221 | 0.804–1.854 | 0.348 |
| Depth of invasion | 1.908 | 1.509–2.412 | <0.001 | 1.309 | 0.906–1.890 | 0.151 |
| Regional lymph node involvement | 1.332 | 1.054–1.682 | 0.016 | 1.034 | 0.691–1.547 | 0.870 |
| Localisation | 1.122 | 0.758–1.661 | 0.566 | – | – | – |
| Immunohistochemical expression of Gpx-1 in cancer tissue | 7.102 | 4.479–11.261 | <0.001 | 2.755 | 1.554–4.883 | 0.001 |
| Angioinvasion | 3.940 | 2.227–6.970 | <0.001 | 0.798 | 0.404–1.579 | 0.518 |
| Expression of PCNA | 20.226 | 10.602–38.586 | <0.001 | 10.219 | 4.841–21.572 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzozowa-Zasada, M.; Piecuch, A.; Bajdak-Rusinek, K.; Janelt, K.; Michalski, M.; Klymenko, O.; Matysiak, N. Immunohistochemical Expression of Glutathione Peroxidase 1 (Gpx-1) as an Independent Prognostic Factor in Colon Adenocarcinoma Patients. Pharmaceuticals 2023, 16, 740. https://doi.org/10.3390/ph16050740
Brzozowa-Zasada M, Piecuch A, Bajdak-Rusinek K, Janelt K, Michalski M, Klymenko O, Matysiak N. Immunohistochemical Expression of Glutathione Peroxidase 1 (Gpx-1) as an Independent Prognostic Factor in Colon Adenocarcinoma Patients. Pharmaceuticals. 2023; 16(5):740. https://doi.org/10.3390/ph16050740
Chicago/Turabian StyleBrzozowa-Zasada, Marlena, Adam Piecuch, Karolina Bajdak-Rusinek, Kamil Janelt, Marek Michalski, Olesya Klymenko, and Natalia Matysiak. 2023. "Immunohistochemical Expression of Glutathione Peroxidase 1 (Gpx-1) as an Independent Prognostic Factor in Colon Adenocarcinoma Patients" Pharmaceuticals 16, no. 5: 740. https://doi.org/10.3390/ph16050740