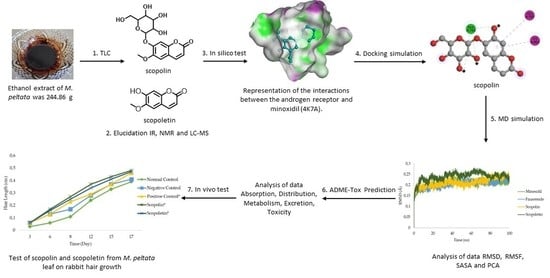
Anti-Alopecia Activity of Coumarin Derivatives Isolated from Merremia peltata Leaves and Computational Study of Their Binding to Androgen Receptors Using Molecular Docking and Molecular Dynamic Simulation
Abstract
:1. Introduction
2. Results
2.1. Elucidation of the Compounds Structure
2.2. Docking Simulation of Minoxidil, Finasteride, and Test Ligands (Scopolin and Scopoletin from M. peltata Leaf)
2.2.1. Preparation of Protein Receptor
2.2.2. Validation of Molecular Docking Method
2.2.3. Docking Simulation
2.2.4. Molecular Dynamic Simulation
2.2.5. ADME-Tox Prediction
2.3. Hair Growth Activity of Scopolin and Scopoletin from M. peltata Leaf
3. Discussion
3.1. Docking Simulation of Minoxidil, Finasteride, and Test Ligands (Scopolin and Scopoletin from M. peltata Leaf)
3.1.1. Preparation of Protein Receptor
3.1.2. Validation of Molecular Docking Method
3.1.3. Docking Simulation
3.1.4. Molecular Dynamic Simulation
3.1.5. ADME-Tox Prediction
3.2. Hair Growth Activity of Compounds Scopolin and Scopoletin Isolated from M. peltata Leaf
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation and Determination
4.3. Extraction
4.4. Extract Purification and Isolation
4.5. Elucidation of the Compounds’ Structures
4.6. Docking Simulation of Minoxidil, Finasteride, and Test Ligands (Compounds Scopolin and Scopoletin from M. peltata Leaf)
4.6.1. Preparation of Ligand Structure
4.6.2. Preparation of Protein Receptor
4.6.3. Validation of the Molecular Docking Method
4.6.4. Docking Simulation
4.6.5. Molecular Dynamic Simulation
4.6.6. Prediction of ADME-Tox
4.7. Hair Growth Activity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DHT | Dihydrotestosterone |
| NMR | Nuclear magnetic resonance |
| LC-MS | Liquid chromatography mass spectrometry |
| ADME-Tox | Absorption, distribution, metabolism, excretion, and toxicology |
| MM-PBSA | Molecular mechanics/Poisson–Boltzmann surface area |
| SASA | Solvent accessible surface area |
| PCA | Principal component analysis |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| DPCs | Dermal papilla cells |
| VLC | Vacuum liquid chromatography |
| KR | Radial chromatography |
| TLC | Thin layer chromatography |
| MD | Molecular dynamics |
| IR | Infrared |
| HR-ESI-TOF | High-resolution electrospray ionization time-of-flight |
| NR3C4 | Nuclear receptor subfamily 3, group C, member 4 |
| Na-CMC | Carboxymethyl cellulose sodium |
| DBE | Double bond equivalence |
| LNCaP | Lymph node carcinoma of the prostate |
| DEPT | Distortion-less enhancement by polarization transfer |
| ANOVA | Analysis of variances |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| MeOH | Methanol |
| CHCl3 | Chloroform |
| MDCK | Madin–Darby kidney cell model |
| PB | Plasma–protein barrier |
References
- Obasi, C.J.; Obasi, I.S.; Okafor, U.C.; Uzoka, I.S. Comparison of anti-dandruff activity of synthetic shampoos and crude plant extracts on dandruff causing isolates. J. Bio. Biochem. 2018, 4, 42–46. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Justine, A.E.; Rodney, S.; Stephen, B.H. Androgenetic Alopecia: Pathogenesis and Potential for Therapy; Cambridge University Press: Cambridge, UK, 2002; pp. 1–11. [Google Scholar]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Ruksiriwanich, W.; Manosroi, W.; Abe, M.; Manosroi, J. In vivo hair growth promotion activity of gel containing niosomes loaded with the Oryza sativa bran fraction (OSF3). Adv. Sci. Lett. 2012, 16, 222–228. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Price, V.H. Different levels of 5α-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J. Investig. Dermatol. 1997, 109, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.; Kotiyal, R.; Chauhan, A.; Mishari, A.; Adhikari, L.; Semalty, A.; Semalty, M. Alopecia and the herbal drugs: An overview of the current status. Adv. Biomed. Pharm. 2015, 2, 246–254. [Google Scholar] [CrossRef]
- Ruslin; Sahidin, I. Identification and determination of traditional medicinal plants in Southeast Sulawesi community at Arboretum Prof. Mahmud Hamundu Haluoleo University. Maj. Farm. Ind. 2008, 19, 101–107. [Google Scholar] [CrossRef]
- Abdurrahman, S.; Ruslin, R.; Hasanah, A.N.; Mustarichie, R.; Ifaya, M. Active Antialopecia Chemical Identification of Merremia peltata Leaves and Computational Study toward Androgen Receptor Using Molecular Docking and Molecular Dynamic Simulation. Sci. World J. 2022, 2022, 1123047. [Google Scholar] [CrossRef]
- Abdurrahman, S.; Ruslin, R.; Hasanah, A.N.; Mustarichie, R. Molecular docking studies and ADME-Tox prediction of phytocompounds from Merremia peltata as a potential anti-alopecia treatment. J. Adv. Pharm. Technol. Res. 2021, 12, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, E.; Pellegrinelli, R.P.; Rizzo, T.R.; Muyskens, M.A. Cryogenic Infrared Action Spectroscopy Fingerprints the Hydrogen Bonding Network in Gas-Phase Coumarin Cations. J. Phys. Chem. A 2020, 124, 9942–9950. [Google Scholar] [CrossRef] [PubMed]
- Ferdinal, N.; Alfajri, R.; Arifin, B. Isolation and Characterization of Scopoletin from The Bark of Fragraea ceilanica thumb and Antioxidants Tests. Int. J. Adv. Sci. Eng. Inf. Tech. 2015, 5, 70–74. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, E.J.; Whang, W.K.; Park, C.H. In vitro and in vivo anti-aging effects of compounds isolated from Artemisia iwayomogi. J. Anal. Sci. Technol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Juliawati, L.D.; Ra’idah, P.N.; Abdurrahman, S.; Hermawati, E.; Alni, A.; Tan, M.I.; Ishikawa, H.; Syah, Y.M. 5,6-Dihydro-α-pyrones from the leaves of Cryptocarya pulchinervia (Lauraceae). J. Nat. Med. 2020, 74, 584–590. [Google Scholar] [CrossRef]
- Mehul, B.; Kishor, D.; Ajay, S. Isolation and structure elucidation of Scopoletin from Ipomoea reniformis (Convolvulaceae). J. App. Pharm. Sci. 2011, 1, 138–144. [Google Scholar]
- Rodney, D.S. Male androgenetic alopecia. JMG 2004, 44, 319–327. [Google Scholar] [CrossRef]
- Dow, S.; Kurt, S.; Robert, H.; William, M.P.; James, E.V.; David, A.W. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo. Clinic. Proceeding 2005, 10, 1316–1322. [Google Scholar] [CrossRef]
- Bayoumy, I.A.; Hameed, E. Effect of oral finasteride on serum androgen levels and androgenetic alopecia in adult men. J. Pan-Arab. Leag. Dermatol. 2007, 18, 37–45. [Google Scholar]
- Nicole, E.R.; Marc, R.A. Medical treatments for male and female patter hair loss. J. Am Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef]
- Guay, A.T. Advances in the management of androgen deficiency in women. Med. Asp. Hum. Sex. 2001, 1, 32–38. [Google Scholar] [CrossRef]
- Ramirez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data. Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Girija, C.R.; Karunakar, P.; Poojari, C.S.; Begum, N.S.; Syed, A.A. Molecular docking studies of curcumin derivatives with multiple protein targets for procarcinogen activating enzyme inhibition, J. J. Proteomics. Bio. Inform. 2010, 3, 200–203. [Google Scholar] [CrossRef]
- Glowacki, E.D.; Vladu, M.I.; Bauer, S. Hydrogen bonds in molecular solids-from biological systems to organic electronics. J. Mater. Chem. B 2013, 1, 3742–3753. [Google Scholar] [CrossRef]
- Sharp, K.A.; Honig, B. Electrostatic interactions in macromolecules: Theory and applications. Annu. Rev. Bio. Phys. Chem. 1990, 19, 301–322. [Google Scholar] [CrossRef]
- Perpina, E.E.; Arnold, A.A.; Baxter, D.; Webb, P. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc. Natl. Acad. Sci. USA 2007, 104, 16074–16079. [Google Scholar] [CrossRef]
- Lins, L.; Brasseur, R. The hydrophobic effect in protein folding. FASEB J. 1995, 9, 535–540. [Google Scholar] [CrossRef]
- Kendel, S.; Westwell, A.D.; McGuigan, C. 7-Substituted umbelliferone derivatives as androgen receptor antagonists for the potential treatment of prostate and breast cancer. Bio. Med. Chem. Let. 2016, 26, 2000–2004. [Google Scholar] [CrossRef]
- Koga, H.; Negishi, M.; Konoshita, M.; Fujii, S.; Mori, S.; Ishigami, M.; Kawachi, E.; Kagechiki, H.; Tanatani, A. Development of androgen-antagonistic coumarinamides with a unique aromatic folded pharmacophore. Int. J. Mol. Sci. 2020, 21, 5584. [Google Scholar] [CrossRef]
- Morikawa, P.; Luo, F.; Manse, Y.; Sugita, H.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, P.; Muraoka, O.; Ninomiya, K. Geranylated Coumarins From Thai Medicinal Plant Mammea siamensis With Testosterone 5α-Reductase Inhibitory Activity. Front. Chem. 2020, 8, 199. [Google Scholar] [CrossRef]
- Pitalokas, D.A.E.; Ramadhan, D.S.F.; Arfan; Chaidir, L.; Fakih, T.M. Docking-based virtual screening and molecular dynamics simulations of quercetin analogs as enoyl-acyl carrier protein reductase (Inh A) inhibitors of Mycobacterium tuberculosis. J. Sci. Pharm. 2021, 89, 20. [Google Scholar] [CrossRef]
- Mannhold, R. (Ed.) Molecular Drug Properties: Measurement and Prediction; Wiley-VHC Verlag: Hoboken, NJ, USA, 2008.
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.J.; Zhang, W.; Xia, K.; Qiao, X.B.; Xu, X.J. ADME evaluation in drug discovery correlation of Caco-2 permeation with simple molecular properties. J. Chem. Inf. Com. Sci. 2004, 44, 1585–1600. [Google Scholar] [CrossRef]
- Wright, D.B. Comparing groups in a before-after design: When t-test and ANCOVA produce different results. Br. J. Educ. Psychol. 2006, 76, 663–675. [Google Scholar] [CrossRef]
- Luo, J.; Chen, M.; Liu, Y.; Xie, H.; Yuan, J.; Zhou, Y.; Ding, J.; Deng, Z.; Li, J. Nature-derived lignan compound VB-1 exerts hair growth-promoting effects by augmenting Wnt/β-Catenin signaling in human dermal papilla cells. PeerJ 2018, 6, e4737. [Google Scholar] [CrossRef]
- Mustarichie, R.; Wicaksono, I.A.; Gozali, D. Anti-alopecia activity of DADAP (Erythrina variegata L.) leaves ethanol extract. J. Pharm. Sci. 2017, 9, 1849–1854. [Google Scholar]
- BIOVIA. Dassault Systèmes. Discovery Studio Visualizer, v21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2021. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 10 December 2021).
- Jain, A.N.; Nicholls, A. Recommendations for evaluation of computational methods. J. Com. Aid. Mol. Des. 2008, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Softw. X 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; State University of New York: New York, NY, USA; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bowers, L.D. High-performance liquid chromatography/mass spectrometry: State of the art for the drug analysis laboratory. Clin. Chem. 1989, 35, 1282–1287. [Google Scholar] [CrossRef]
- Essmann, U.L.; Perera, M.; Berkowitz, T.; Darden, H.; Lee, L.P. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Silva, S.D.; Alan, W.; Wim, F.; Vranken. ACPYPE—Antechamber Python Parser Interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Mark, P.; Lennart, N. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. 2001, 43, 9954–9960. [Google Scholar] [CrossRef]
- Tanaka, S.; Saito, M.; Tabata, M. Bioassay of crude drugs for hair growth promoting activity in mice by a new simple method. Plant. Med. 1980, 40, 84–90. [Google Scholar] [CrossRef]
- Federer, W. Experimental Design, Theory, and Application; MacMillan: New York, NY, USA, 1963. [Google Scholar]








| No | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δ 1H (500 MHz) | δ 13C (125 MHz) | δ 1H (500 MHz) | δ 13C (125 MHz) | |
| 2 | - | 163.5 | - | 164.2 |
| 3 | 6.19 (1H, d, J = 11.5) | 114.5 | 6.14 (1H, d, J = 11.5) | 113.0 |
| 4 | 7.85 (1H, d, J = 11.5) | 145.6 | 7.81 (1H, d, J = 11.5) | 146.1 |
| 5 | 7.10 (1H, s) | 105.1 | 7.16 (1H, s) | 109.9 |
| 6 | - | 150.6 | - | 151.4 |
| 7 | - | 148.3 | - | 147.3 |
| 8 | 6.76 (1H, s) | 102.2 | 6.76 (1H, s) | 104.0 |
| 9 | - | 151.8 | - | 152.9 |
| 10 | - | 110.7 | - | 112.4 |
| 11 | 3.90 (3H, s) | 57.0 | 3.87 (3H, s) | 56.7 |
| 1′ | 5.26 (1H, d, J = 7.5) | 113.9 | ||
| 2′ | 3.48 (1H, t, J = 9) | 74.7 | ||
| 3′ | 3.43 (1H, t, J = 9) | 77.8 | ||
| 4′ | 3.55 (1H, t, J = 9,5) | 71.1 | ||
| 5′ | 3.58 (1H, dd, J = 12; 5.5) | 78.4 | ||
| 6′ | 3.22 (2H, m) | 62.4 | ||
| Compound | Binding Energy (kcal/mol) | Hydrogen Bond Distance (Ǻ) | Hydrogen Bonds | Nearest Amino Acid Residue(s) |
|---|---|---|---|---|
| Scopolin | −4.51 | 1.86 | GLU793 | LYS861, LUE797, LEU862 |
| Scopoletin | −4.65 | 1.80 | SER865 | LEU862, LYS861, GLU793 |
| Ligand | van der Waals Energy (KJ/mol) | Electrostatic Energy (KJ/mol) | Polar Solvation Energy (KJ/mol) | SASA Energy (KJ/mol) | Total Binding Energy (KJ/mol) |
|---|---|---|---|---|---|
| Minoxidil | −134.036 +/− 9.802 | −9.222 +/− 12.200 | 104.959 +/− 13.690 | −13.511 +/− 0.592 | −51.810 +/− 14.266 |
| Finasteride | −112.958 +/− 17.378 | −38.599 +/− 12.965 | 102.071 +/− 23.052 | −13.380 +/− 1.690 | −62.867 +/− 14.004 |
| Scopolin | −96.419 +/− 15.585 | −100.845 +/− 23.749 | 171.825 +/− 29.130 | −11.966 +/− 1.147 | −37.404 +/− 16.986 |
| Scopoletin | −16.785 +/− 34.408 | −4.731 +/− 12.402 | 293.019 +/− 1393.615 | −1.878 +/− 3.788 | 269.626 +/− 1398.112 |
| Compound | Absorption | Distribution | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Minoxidil | −2.871 | 0.653 | 94.641 | −2.798 | 0.142 | 0.773 | −0.951 | −3.471 |
| Finasteride | −5.148 | 1.269 | 93.742 | −3.463 | −0.185 | 0.01 | −0.18 | −1.821 |
| Scopolin | −2.21 | 0.377 | 48.119 | −2.822 | −0.611 | 0.397 | −1.286 | −3.954 |
| Scopoletin | −2.504 | 1.184 | 95.277 | −2.944 | 0.034 | 0.363 | −0.299 | −2.32 |
| Compound | Metabolism | Excretion | Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Minoxidil | No | No | 0.275 | No | −0.359 | No | No | 2.286 | Yes | No | 3.516 |
| Finasteride | Yes | Yes | 0.38 | No | −1.355 | No | No | 2.424 | Yes | No | 0.638 |
| Scopolin | No | No | 0.716 | No | 0.393 | No | No | 3.756 | No | No | 4.198 |
| Scopoletin | No | No | 0.73 | No | 0.614 | No | No | 1.378 | No | No | 1.614 |
| No. | IUPAC Name | Structure |
|---|---|---|
| 1. | Natural ligand Minoxidil |  |
| 2. | Reference ligand finasteride |  |
| 3. | Scopolin |  |
| 4. | Scopoletin |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdurrahman, S.; Ruslin, R.; Hasanah, A.N.; Ifaya, M.; Mustarichie, R. Anti-Alopecia Activity of Coumarin Derivatives Isolated from Merremia peltata Leaves and Computational Study of Their Binding to Androgen Receptors Using Molecular Docking and Molecular Dynamic Simulation. Pharmaceuticals 2023, 16, 669. https://doi.org/10.3390/ph16050669
Abdurrahman S, Ruslin R, Hasanah AN, Ifaya M, Mustarichie R. Anti-Alopecia Activity of Coumarin Derivatives Isolated from Merremia peltata Leaves and Computational Study of Their Binding to Androgen Receptors Using Molecular Docking and Molecular Dynamic Simulation. Pharmaceuticals. 2023; 16(5):669. https://doi.org/10.3390/ph16050669
Chicago/Turabian StyleAbdurrahman, Syawal, Ruslin Ruslin, Aliya Nur Hasanah, Mus Ifaya, and Resmi Mustarichie. 2023. "Anti-Alopecia Activity of Coumarin Derivatives Isolated from Merremia peltata Leaves and Computational Study of Their Binding to Androgen Receptors Using Molecular Docking and Molecular Dynamic Simulation" Pharmaceuticals 16, no. 5: 669. https://doi.org/10.3390/ph16050669