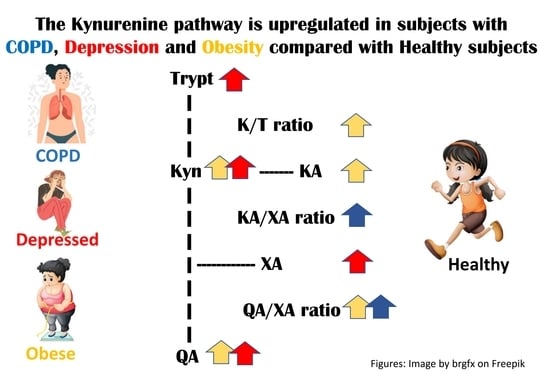
The Kynurenine Pathway in Healthy Subjects and Subjects with Obesity, Depression and Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data, Material and Study Design
2.2. Variables
2.3. Statistics
3. Results
3.1. Participants’ Characteristics
3.2. The KP Metabolites and Differences between the Groups
3.3. The Associations between the KP and the Predictors BMI, Smoking, Diabetes, and CRP
3.4. Differences in the KP between the Groups of Participants after Adjusting for Age, Sex, BMI, Smoking and CRP, All at the Same Time
- Obese compared with Healthy: Kyn: 49 (−13; 112); p = 0.120. KA: 1.1 (−1.7; 3.9); p = 0.448. QA: 20 (1.6; 39); p = 0.034. K/T ratio: 2.9 (−1.8; 7.6); p = 0.229. QA/XA ratio: 2.2 (−4.4; 8.7); p = 0.510.
- Depressed compared with Healthy: K/T ratio: 6.4 (2.3; 10.6); p = 0.002.
- COPD compared with Healthy: No changes.
- Depressed compared with Obese: Trypt: 1312 (−95; 2719); p = 0.067. XA: 0.55 (−0.08; 1.2); p = 0.086.
- Depressed compared with COPD: QA/XA ratio: −10 (−19; −1.9); p = 0.017.
- Obese compared with COPD: QA/XA ratio: −13 (−23; −4); p = 0.006.
4. Discussion
4.1. Differences in the KP between the Healthy Group and the Groups with Diseases (Section 3.2, Table 2)
4.2. Differences in the KP between the Groups with Diseases (Section 3.2, Table 3)
4.3. Associations between the KP Metabolites and One-By-One of the Covariates (Section 3.3, Table 4)
4.4. Differences in the KP between the Healthy Group and the Groups with Diseases after Adjusting for Age, Sex, BMI, Smoking and CRP, All at the Same Time (Section 3.4)
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed.) 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [Green Version]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Zhang, H.; Tian, L.; Gui, S.; Yu, Y.; Chen, X.; Chen, Y.; Yang, L.; Ran, Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2021, 26, 4265–4276. [Google Scholar] [CrossRef] [Green Version]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef]
- Gosker, H.R.; Clarke, G.; de Theije, C.C.; Cryan, J.F.; Schols, A. Impaired Skeletal Muscle Kynurenine Metabolism in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Suresh, B.; Lim, M.N.; Hong, S.H.; Kim, K.S.; Song, H.E.; Lee, H.Y.; Yoo, H.J.; Kim, W.J. Metabolomics Reveals Dysregulated Sphingolipid and Amino Acid Metabolism Associated with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.H.; Gao, Z.; Wang, Y.; Wang, X.; et al. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun. 2022, 13, 3489. [Google Scholar] [CrossRef]
- Athnaiel, O.; Ong, C.; Knezevic, N.N. The Role of Kynurenine and Its Metabolites in Comorbid Chronic Pain and Depression. Metabolites 2022, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Hestad, K. The Peripheral Kynurenine Pathway and Psychosomatic Comorbidity in Subjects with Morbid Obesity Undergoing Bariatric Surgery. Appl. Sci. 2022, 12, 2648. [Google Scholar] [CrossRef]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017, 8, 1561. [Google Scholar] [CrossRef] [Green Version]
- Molmen, K.S.; Hammarstrom, D.; Falch, G.S.; Grundtvig, M.; Koll, L.; Hanestadhaugen, M.; Khan, Y.; Ahmad, R.; Malerbakken, B.; Rodolen, T.J.; et al. Chronic obstructive pulmonary disease does not impair responses to resistance training. J. Transl. Med. 2021, 19, 292. [Google Scholar] [CrossRef]
- Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J. Clin. Med. 2021, 10, 2484. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Favennec, M.; Hennart, B.; Verbanck, M.; Pigeyre, M.; Caiazzo, R.; Raverdy, V.; Verkindt, H.; Leloire, A.; Guillemin, G.J.; Yengo, L.; et al. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS ONE 2016, 11, e0158051. [Google Scholar] [CrossRef] [Green Version]
- Taleb, O.; Maammar, M.; Klein, C.; Maitre, M.; Mensah-Nyagan, A.G. A Role for Xanthurenic Acid in the Control of Brain Dopaminergic Activity. Int. J. Mol. Sci. 2021, 22, 6974. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol. Neurobiol. 2015, 52, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theofylaktopoulou, D.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygard, O.; Eussen, S.J. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Neurauter, G.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Fuchs, D.; Miller, A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 2003, 54, 906–914. [Google Scholar] [CrossRef]
- Hestad, K.A.; Aukrust, P.; Tønseth, S.; Reitan, S.K. Depression has a Strong Relationship to Alterations in the Immune, Endocrine and Neural System. Curr. Psychiatry Rev. 2009, 5, 287–297. [Google Scholar] [CrossRef]
- Naz, S.; Bhat, M.; Stahl, S.; Forsslund, H.; Skold, C.M.; Wheelock, A.M.; Wheelock, C.E. Dysregulation of the Tryptophan Pathway Evidences Gender Differences in COPD. Metabolites 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- van der Vaart, H.; Postma, D.S.; Timens, W.; ten Hacken, N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lin, H.; Zou, M.; Yuan, Q.; Huang, Z.; Pan, X.; Zhang, W. Nicotine in Inflammatory Diseases: Anti-Inflammatory and Pro-Inflammatory Effects. Front. Immunol. 2022, 13, 826889. [Google Scholar] [CrossRef]
- Klimek, V.; Zhu, M.Y.; Dilley, G.; Konick, L.; Overholser, J.C.; Meltzer, H.Y.; May, W.L.; Stockmeier, C.A.; Ordway, G.A. Effects of long-term cigarette smoking on the human locus coeruleus. Arch. Gen. Psychiatry 2001, 58, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics (No. If Less than 320) | Healthy n = 108 | Obese n = 141 | Depressed n = 49 | COPD n = 22 | Statistics (p-Values) |
|---|---|---|---|---|---|
| Age (years) † | 57.9 (11.6) | 43.0 (8.7) | 45.3 (14.6) | 69.3 (5.1) | <0.001 * |
| Sex (male) | 46 (43%) | 31 (22%) | 23 (47%) | 14 (64%) | <0.001 ** |
| BMI (kg/m2) (n = 315) | 27.6 (5.4) | 42.1 (3.8) | 25.8 (4.4) | 24.7 (5.0) | <0.001 * |
| Daily smoker (n = 315) | 1 (1%) | 25 (18%) | 23 (48%) | 4 (20%) | <0.001 ** |
| Diabetes (n = 232) | 1 (2%) | 26 (19%) | 2 (4%) | N.A. | 0.002 ** |
| CRP (mg/L) (n = 313) | 2.6 (5.8) | 7.1 (6.3) | 2.1 (2.6) | 5.0 (7.7) | <0.001 * |
| Dependent Variables | Independent Variables | |||||
|---|---|---|---|---|---|---|
| KP Metabolites | KP Metabolites Healthy Group (ng/mL) | Age (Years) | Sex (Male) | Obese Compared with Healthy | Depressed Compared with Healthy | COPD Compared with Healthy |
| Tryptophan (Trypt) | 12379 (1978) | −6 (−32; 19) p = 0.626 | 909 (317; 1500) p = 0.003 | 342 (−400; 1084) p = 0.365 | 1606 (705; 2507) p = 0.001 | 125 (−1058; 1308) p = 0.836 |
| Kynurenine (Kyn) | 440 (106) | 2.2 (0.9; 3.6) p = 0.002 | 17 (−15; 49) p = 0.298 | 85 (45; 125) p < 0.001 | 96 (48; 145) p < 0.001 | 37 (−26; 101) p = 0.248 |
| Kynurenic acid (KA) | 12.8 (4.2) | 0.11 (0.04; 0.17) p = 0.001 | 0.35 (−1.09; 1.78) p = 0.635 | 3.10 (1.30; 4.90) p = 0.001 | 1.11 (−1.07; 3.29) p = 0.318 | −0.42 (−3.28; 2.45) p = 0.774 |
| Quinolinic acid (QA) | 83 (30) | 0.43 (0.006; 0.86) p = 0.047 | 4.9 (−4.9; 14.6) p = 0.325 | 35 (23; 48) p < 0.001 | 23 (8; 38) p = 0.003 | 15 (−4; 35) p = 0.116 |
| Xanthurenic acid (XA) | 5.1 (0.9) | 0.00 (−0.01; 0.01) p = 0.878 | 0.37 (0.11; 0.63) p = 0.006 | 0.18 (−0.15; 0.50) p = 0.293 | 0.70 (0.30; 1.10) p = 0.001 | −0.23 (−0.75; 0.29) p = 0.388 |
| K/T ratio × 1000 | 36 (9) | 0.19 (0.08; 0.30) p = 0.001 | −0.93 (−3.4; 1.6) p = 0.462 | 6.1 (2.9; 9.2) p < 0.001 | 3.0 (−0.8; 6.8) p = 0.118 | 3.5 (−1.5; 8.5) p = 0.169 |
| KA/Kyn ratio × 1000 | 30 (8) | 0.04 (−0.07; 0.14) p = 0.504 | −0.79 (−3.3; 1.7) p = 0.530 | 0.58 (−2.52; 3.69) p = 0.712 | −3.69 (−7.46; 0.09) p = 0.056 | −2.72 (−7.68; 2.24) p = 0.281 |
| KA/QA ratio × 1000 | 170 (70) | 0.29 (−0.42; 1.01) p =0.423 | 0.28 (−16.2; 16.7) p = 0.973 | −23.2 (−43.8; −2.6) p = 0.028 | −25.6 (−50.6; −0.5) p = 0.045 | −15.3 (−48.2; 17.6) p = 0.361 |
| KA/XA ratio | 2.5 (0.9) | 0.02 (−0.003; 0.04) p = 0.085 | 0.09 (−0.43; 0.62) p = 0.773 | 0.55 (−0.11; 1.21) p = 0.101 | −0.11 (−0.91; 0.69) p = 0.788 | 1.52 (0.46; 2.57) p = 0.005 |
| QA/XA ratio | 16.6 (7.2) | 0.07 (−0.08; 0.21) p = 0.344 | 0.36 (−2.96; 3.69) p = 0.830 | 6.4 (2.2; 10.5) p = 0.003 | 1.6 (−3.5; 6.7) p = 0.537 | 12.6 (6.0; 19.3) p < 0.001 |
| KP Metabolites | Depressed Compared with Obese | Depressed Compared with COPD | Obese Compared with COPD |
|---|---|---|---|
| Tryptophan | 1263 (440; 2087) p = 0.003 | 1481 (82; 2879) p = 0.038 | 217 (−1111; 1546) p = 0.748 |
| Kynurenine | 11 (−33; 56) p = 0.617 | 58 (−17; 134) p = 0.126 | 47 (−24; 119) p = 0.193 |
| Kynurenic acid | −2.0 (−4.0; 0.01) p = 0.051 | 1.5 (−1.9; 4.9) p = 0.376 | 3.5 (0.3; 6.7) p = 0.032 |
| Quinolinic acid | −12.7 (−26.3; 0.9) p = 0.067 | 7.1 (−15.9; 30.2) p = 0.544 | 19.8 (−2.1; 41.7) p = 0.076 |
| Xanthurenic acid | 0.53 (0.16; 0.89) p = 0.005 | 0.93 (0.31; 1.55) p = 0.003 | 0.41 (−0.18; 0.99) p = 0.176 |
| K/T ratio × 1000 | −3.1 (−6.5; 0.4) p = 0.086 | −0.4 (−6.3; 5.4) p = 0.876 | 2.6 (−3.0; 8.2) p = 0.367 |
| KA/Kynurenine ratio × 1000 | −4.3 (−7.7; −0.8) p = 0.015 | −1.0 (−6.8; 4.9) p = 0.746 | 3.3 (−2.2; 8.9) p = 0.243 |
| KA/QA ratio × 1000 | −2.4 (−25.3; 20.5) p = 0.838 | −10.3 (−49.1; 28.6) p = 0.604 | −7.9 (−44.8; 29.0) p = 0.675 |
| KA/XA ratio | −0.7 (−1.4; 0.1) p = 0.076 | −1.6 (−2.9; −0.4) p = 0.011 | −1.0 (−2.1; 0.2) p = 0.110 |
| QA/XA ratio | −4.8 (−9.4; −0.14) p = 0.043 | −11.0 (−18.9; −3.2) p = 0.006 | −6.3 (−13.7; 1.2) p = 0.100 |
| Dependent Variables | Independent Variables | |||
|---|---|---|---|---|
| KP Metabolites | BMI (kg/m2) | Smoking | Diabetes | CRP |
| Tryptophan (Trypt) | −51 (−99; 3) p = 0.038 | |||
| Kynurenine (Kyn) | −46 (−90; −3) p = 0.037 | 3.7 (1.1; 6.3) p = 0.005 | ||
| Kynurenic acid (KA) | 0.21 (0.05; 0.36) p = 0.008 | −3.1 (−5.7; −0.6) p = 0.014 | ||
| Quinolinic acid (QA) | 1.6 (0.6; 2.6) p = 0.003 | −21 (−35; −9) p = 0.001 | 1.3 (0.5; 2.1) p = 0.001 | |
| K/T ratio × 1000 | 0.3 (0.02; 0.5) p = 0.037 | −4.1 (−7.5; −0.7) p = 0.019 | 0.55 (0.36; 0.75) p < 0.001 | |
| KA/Kyn × 1000 | −0.2 (−0.4; 0.03) p = 0.025 | |||
| KA/QA ratio × 1000 | −1.8 (−3.1; −0.5) p = 0.007 | |||
| KA/XA ratio | −0.6 (−1.1; −0.2) p = 0.006 | |||
| QA/XA ratio | 0.42 (0.06; 0.77) p = 0.022 | −5.5 (−10.0; −0.9) p = 0.018 | −3.8 (−7.2; −0.4) p =0.031 | 0.37 (0.10; 0.64) p = 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farup, P.G.; Hamarsland, H.; Mølmen, K.S.; Ellefsen, S.; Hestad, K. The Kynurenine Pathway in Healthy Subjects and Subjects with Obesity, Depression and Chronic Obstructive Pulmonary Disease. Pharmaceuticals 2023, 16, 351. https://doi.org/10.3390/ph16030351
Farup PG, Hamarsland H, Mølmen KS, Ellefsen S, Hestad K. The Kynurenine Pathway in Healthy Subjects and Subjects with Obesity, Depression and Chronic Obstructive Pulmonary Disease. Pharmaceuticals. 2023; 16(3):351. https://doi.org/10.3390/ph16030351
Chicago/Turabian StyleFarup, Per G., Håvard Hamarsland, Knut Sindre Mølmen, Stian Ellefsen, and Knut Hestad. 2023. "The Kynurenine Pathway in Healthy Subjects and Subjects with Obesity, Depression and Chronic Obstructive Pulmonary Disease" Pharmaceuticals 16, no. 3: 351. https://doi.org/10.3390/ph16030351