Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas gingivalis Lipopolysaccharide
Abstract
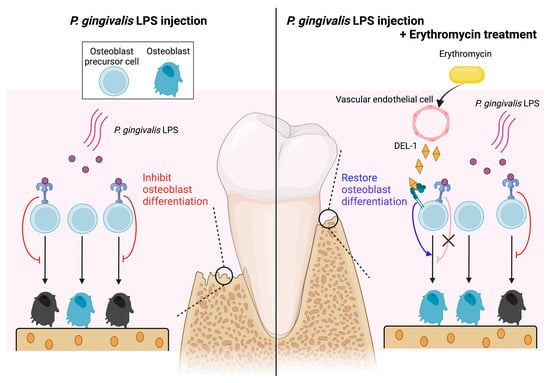
:1. Introduction
2. Results
2.1. ERM Ameliorated P. gingivalis LPS-Induced Decrease in Mineral Nodule Formation in MC3T3 Cells by Promoting Osteoblast Differentiation
2.2. ERM Significantly Suppressed Periodontal Bone Loss Induced by P. gingivalis LPS
2.3. ERM Recovered the Expression of Osteoblast Differentiation-Related Factors Suppressed by P. gingivalis LPS
2.4. ERM Rescued DEL-1 Expression Reduced by P. gingivalis LPS
2.5. ERM Enhanced Alkaline Phosphatase (ALP) Activity Attenuated by P. gingivalis LPS
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Murine Model
4.3. Histological Analysis
4.4. Osteoblastic Progenitors
4.5. Osteogenic Differentiation Assay
4.6. Quantitative Real-Time PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontology 2020, 82, 78–92. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, J.S.; Ramadan, D.; de Paiva Goncalves, V.; Maquera-Huacho, P.M.; Assis, R.P.; Lima, T.F.O.; Brunetti, I.L.; Spolidorio, D.M.P.; Cesar, T.; Manthey, J.A.; et al. Impact of citrus flavonoid supplementation on inflammation in lipopolysaccharide-induced periodontal disease in mice. Food Funct. 2021, 12, 5007–5017. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-kappaB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Yonezawa, D.; Nagai, K.; Ochiai, A.; Taniguchi, M.; Tabeta, K.; Maeda, T.; et al. Peptides from rice endosperm protein restrain periodontal bone loss in mouse model of periodontitis. Arch. Oral Biol. 2019, 98, 132–139. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Yonezawa, D.; Kunitomo, E.; Tabeta, K.; Terao, Y. Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice. Arch. Oral Biol. 2020, 112, 104679. [Google Scholar] [CrossRef]
- Vazquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Sole-Violan, J.; Martin-Loeches, I. Macrolide therapy of pneumonia: Is it necessary, and how does it help? Curr. Opin. Infect. Dis. 2016, 29, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Parsad, D.; Pandhi, R.; Dogra, S. A guide to selection and appropriate use of macrolides in skin infections. Am. J. Clin. Dermatol. 2003, 4, 389–397. [Google Scholar] [CrossRef]
- Jin, C.; Gibani, M.M.; Pennington, S.H.; Liu, X.; Ardrey, A.; Aljayyoussi, G.; Moore, M.; Angus, B.; Parry, C.M.; Biagini, G.A.; et al. Treatment responses to Azithromycin and Ciprofloxacin in uncomplicated Salmonella Typhi infection: A comparison of Clinical and Microbiological Data from a Controlled Human Infection Model. PLoS Negl. Trop. Dis. 2019, 13, e0007955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagari, R.M.; Frazzoni, L.; Marasco, G.; Fuccio, L.; Bazzoli, F. Treatment of Helicobacter pylori infection: A clinical practice update. Minerva Med. 2021, 112, 281–287. [Google Scholar] [CrossRef]
- O’Rourke, V.J. Azithromycin as an adjunct to non-surgical periodontal therapy: A systematic review. Aust. Dent. J. 2017, 62, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, T.D.Y.; Saris, A.; Schultz, M.J.; van der Poll, T. Immunomodulation by macrolides: Therapeutic potential for critical care. Lancet Respir. Med. 2020, 8, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrada, A.C.; Azuma, M.M.; Furusho, H.; Hirai, K.; Xu, S.; White, R.R.; Sasaki, H. Immunomodulation Mediated by Azithromycin in Experimental Periapical Inflammation. J. Endod. 2020, 46, 1648–1654. [Google Scholar] [CrossRef]
- Alenezi, A.; Naito, Y.; Terukina, T.; Prananingrum, W.; Jinno, Y.; Tagami, T.; Ozeki, T.; Galli, S.; Jimbo, R. Controlled release of clarithromycin from PLGA microspheres enhances bone regeneration in rabbit calvaria defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, B.; Wang, B.; Bu, W.; Zhao, L.; Liu, J.; Meng, L.; Wang, L.; Xin, Y.; Wang, D.; et al. Rapamycin promotes osteogenesis under inflammatory conditions. Mol. Med. Rep. 2017, 16, 8923–8929. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Hirayama, S.; Isono, T.; Sasagawa, K.; Yonezawa, D.; Takahashi, N.; Oda, M.; et al. Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics 2021, 10, 312. [Google Scholar] [CrossRef]
- Maekawa, T.; Tamura, H.; Domon, H.; Hiyoshi, T.; Isono, T.; Yonezawa, D.; Hayashi, N.; Takahashi, N.; Tabeta, K.; Maeda, T.; et al. Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1. JCI Insight 2020, 5, e136706. [Google Scholar] [CrossRef]
- Shin, J.; Maekawa, T.; Abe, T.; Hajishengallis, E.; Hosur, K.; Pyaram, K.; Mitroulis, I.; Chavakis, T.; Hajishengallis, G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 2015, 7, 307ra155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuh, D.Y.; Maekawa, T.; Li, X.; Kajikawa, T.; Bdeir, K.; Chavakis, T.; Hajishengallis, G. The secreted protein DEL-1 activates a beta3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J. Biol. Chem. 2020, 295, 7261–7273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lunar Silva, I.; Cascales, E. Molecular Strategies Underlying Porphyromonas gingivalis Virulence. J. Mol. Biol. 2021, 433, 166836. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Bandow, K.; Maeda, A.; Kakimoto, K.; Kusuyama, J.; Shamoto, M.; Ohnishi, T.; Matsuguchi, T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 2010, 402, 755–761. [Google Scholar] [CrossRef]
- Wang, Y.H.; Nemati, R.; Anstadt, E.; Liu, Y.; Son, Y.; Zhu, Q.; Yao, X.; Clark, R.B.; Rowe, D.W.; Nichols, F.C. Serine dipeptide lipids of Porphyromonas gingivalis inhibit osteoblast differentiation: Relationship to Toll-like receptor 2. Bone 2015, 81, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Xing, Q.; Ye, Q.; Fan, M.; Zhou, Y.; Xu, Q.; Sandham, A. Porphyromonas gingivalis lipopolysaccharide inhibits the osteoblastic differentiation of preosteoblasts by activating Notch1 signaling. J. Cell. Physiol. 2010, 225, 106–114. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Nelea, V.; Chicatun, F.; Chien, Y.C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.N.; Kaartinen, M.T.; Vali, H.; Tecklenburg, M.M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, C.; Hu, Y.; Xia, X.; Liao, Y.; Zhang, J.; Chen, H.; Lu, W.; Zhou, W.; Song, Z. New Application of Psoralen and Angelicin on Periodontitis With Anti-bacterial, Anti-inflammatory, and Osteogenesis Effects. Front. Cell. Infect. Microbiol. 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, X.; Qiu, C.; Shen, H.; Zhang, H.; He, Z.; Song, Z.; Zhou, W. The imbalance of Th17/Treg via STAT3 activation modulates cognitive impairment in P. gingivalis LPS-induced periodontitis mice. J. Leukoc. Biol. 2021, 110, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lai, C.H.; Kuo, C.H.; Chang, B.I.; Wu, H.L.; Cheng, T.L. Recombinant thrombomodulin lectin-like domain attenuates Porphyromonas gingivalis lipopolysaccharide-induced osteoclastogenesis and periodontal bone resorption. J. Periodontol. 2021, 92, 1622–1634. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, L.; Wang, J.G.; Wang, F.; Yang, X.K.; Zhang, F.H.; Song, J.L.; Ma, X.Y.; Cheng, Q.; Song, G.H. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef]
- Liu, Y.H.; Huang, D.; Li, Z.J.; Li, X.H.; Wang, X.; Yang, H.P.; Tian, S.P.; Mao, Y.; Liu, M.F.; Wang, Y.F.; et al. Toll-like receptor-4-dependence of the lipopolysaccharide-mediated inhibition of osteoblast differentiation. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Muniz, F.W.; de Oliveira, C.C.; de Sousa Carvalho, R.; Moreira, M.M.; de Moraes, M.E.; Martins, R.S. Azithromycin: A new concept in adjuvant treatment of periodontitis. Eur. J. Pharm. 2013, 705, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, R.; Deng, H.; Laohachai, M.N. Azithromycin in periodontal treatment: More than an antibiotic. J. Periodontal. Res. 2012, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; du Bois, A.H.; Gannon, S.; Haynes, D.R.; Hirsch, R.S. Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis. Inflammopharmacology 2013, 21, 321–338. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, Y.S.; Choi, E.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Subantibiotic dose of azithromycin attenuates alveolar bone destruction and improves trabecular microarchitectures in a rat model of experimental periodontitis: A study using micro-computed tomography. Int. Immunopharmacol. 2017, 47, 212–217. [Google Scholar] [CrossRef]
- Steel, H.C.; Theron, A.J.; Cockeran, R.; Anderson, R.; Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat. Inflamm. 2012, 2012, 584262. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Barber, J.; Cikos, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Kamemoto, A.; Ara, T.; Hattori, T.; Fujinami, Y.; Imamura, Y.; Wang, P.L. Macrolide antibiotics like azithromycin increase lipopolysaccharide-induced IL-8 production by human gingival fibroblasts. Eur. J. Med. Res. 2009, 14, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Nagano, T.; Yamaguchi, T.; Kajiyama, S.; Suzuki, T.; Matsushima, Y.; Yashima, A.; Shirakawa, S.; Gomi, K. Effect of Azithromycin on Proinflammatory Cytokine Production in Gingival Fibroblasts and the Remodeling of Periodontal Tissue. J. Clin. Med. 2020, 10, 99. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhao, J.; Miao, W.; Ye, T.; Chen, A. Resveratrol Attenuates Lipopolysaccharides (LPS)-Induced Inhibition of Osteoblast Differentiation in MC3T3-E1 Cells. Med. Sci. Monit. 2018, 24, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, R.J.; Jang, K.; Zhou, X.L.; Liu, Y.Z. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/beta-Catenin Pathways in MC3T3-E1 Cells. Cell. Physiol. Biochem. 2017, 43, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hao, W.; Wang, X.; Su, H. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J. Toxicol. Sci. 2016, 41, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Bao, X.F.; Hu, M.; Yu, W.X. Effects of Porphyromonas gingivalis lipopolysaccharide on osteoblast-osteoclast bidirectional EphB4-EphrinB2 signaling. Exp. Ther. Med. 2014, 7, 80–84. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Hashimoto, M.; Takeuchi, O.; Kurita, T.; Yoshikai, Y.; Miyake, K.; Akira, S. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 2002, 14, 1325–1332. [Google Scholar] [CrossRef]
- Kassem, A.; Lindholm, C.; Lerner, U.H. Toll-Like Receptor 2 Stimulation of Osteoblasts Mediates Staphylococcus Aureus Induced Bone Resorption and Osteoclastogenesis through Enhanced RANKL. PLoS ONE 2016, 11, e0156708. [Google Scholar] [CrossRef] [Green Version]
- Kassem, A.; Henning, P.; Lundberg, P.; Souza, P.P.; Lindholm, C.; Lerner, U.H. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-kappaB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. J. Biol. Chem. 2015, 290, 20147–20158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Li, H.; Zhang, J.; Zhang, X.; Xia, X.; Qiu, C.; Liao, Y.; Chen, H.; Song, Z.; Zhou, W. Periodontitis Induced by P. gingivalis-LPS Is Associated With Neuroinflammation and Learning and Memory Impairment in Sprague-Dawley Rats. Front. Neurosci. 2020, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Acid sphingomyelinase deficiency exacerbates LPS-induced experimental periodontitis. Oral Dis. 2020, 26, 637–646. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Maekawa, T.; Hiyoshi, T.; Terao, Y. Analysis of Experimental Ligature-Induced Periodontitis Model in Mice. Methods Mol. Biol. 2021, 2210, 237–250. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J.; Graves, D.T. The enduring importance of animal models in understanding periodontal disease. Virulence 2015, 6, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.T.; Fine, D.; Teng, Y.T.A.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [Green Version]
- Hidai, C.; Zupancic, T.; Penta, K.; Mikhail, A.; Kawana, M.; Quertermous, E.E.; Aoka, Y.; Fukagawa, M.; Matsui, Y.; Platika, D.; et al. Cloning and characterization of developmental endothelial locus-1: An embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998, 12, 21–33. [Google Scholar] [CrossRef]
- Chavakis, E.; Choi, E.Y.; Chavakis, T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009, 102, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Colamatteo, A.; Kalafati, L.; Kajikawa, T.; Wang, H.; Lim, J.H.; Bdeir, K.; Chung, K.J.; Yu, X.; Fusco, C.; et al. The DEL-1/beta3 integrin axis promotes regulatory T cell responses during inflammation resolution. J. Clin. Investig. 2020, 130, 6261–6277. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. DEL-1: A potential therapeutic target in inflammatory and autoimmune disease? Expert Rev. Clin. Immunol. 2021, 17, 549–552. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. DEL-1-Regulated Immune Plasticity and Inflammatory Disorders. Trends Mol. Med. 2019, 25, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Li, X.; Mitroulis, I.; Grosser, D.; Kajikawa, T.; Wang, B.; Grzybek, M.; von Renesse, J.; Czogalla, A.; Troullinaki, M.; et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019, 20, 40–49. [Google Scholar] [CrossRef]
- Loi, F.; Cordova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Aspenberg, P. Drugs and fracture repair. Acta Orthop. 2005, 76, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, T.; Hosur, K.; Abe, T.; Kantarci, A.; Ziogas, A.; Wang, B.; Van Dyke, T.E.; Chavakis, T.; Hajishengallis, G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat. Commun. 2015, 6, 8272. [Google Scholar] [CrossRef]
- Page, R.C.; Altman, L.C.; Ebersole, J.L.; Vandesteen, G.E.; Dahlberg, W.H.; Williams, B.L.; Osterberg, S.K. Rapidly progressive periodontitis. A distinct clinical condition. J. Periodontol. 1983, 54, 197–209. [Google Scholar] [CrossRef]
- Silva-Costa, C.; Friaes, A.; Ramirez, M.; Melo-Cristino, J. Macrolide-resistant Streptococcus pyogenes: Prevalence and treatment strategies. Expert Rev. Anti-Infect. Ther. 2015, 13, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Lanata, M.M.; Wang, H.; Everhart, K.; Moore-Clingenpeel, M.; Ramilo, O.; Leber, A. Macrolide-Resistant Mycoplasma pneumoniae Infections in Children, Ohio, USA. Emerg. Infect. Dis. 2021, 27, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Belanger, A.E.; Shryock, T.R. Macrolide-resistant Campylobacter: The meat of the matter. J. Antimicrob. Chemother. 2007, 60, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kimura, O.; Domon, H.; Maekawa, T.; Yonezawa, D.; Terao, Y. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children with acute otitis media in Japan from 2014 to 2017. J. Infect. Chemother. 2019, 25, 229–232. [Google Scholar] [CrossRef]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; Leon, R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral Dis. 2019, 25, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Ishinaga, H.; Suzuki, S.; Sugawara, A.; Sunazuka, T.; Omura, S.; Jono, H.; Takeuchi, K. Effects of a novel nonantibiotic macrolide, EM900, on cytokine and mucin gene expression in a human airway epithelial cell line. Pharmacology 2011, 88, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, H.; Takahashi, K.; Tashiro, H.; Kato, G.; Noguchi, Y.; Kurata, K.; Omura, S.; Kimura, S.; Sunazuka, T.; Sueoka-Aragane, N. The non-antibiotic macrolide EM900 attenuates HDM and poly(I:C)-induced airway inflammation with inhibition of macrophages in a mouse model. Inflamm. Res. 2020, 69, 139–151. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, H.; Maekawa, T.; Domon, H.; Sirisereephap, K.; Isono, T.; Hirayama, S.; Hiyoshi, T.; Sasagawa, K.; Takizawa, F.; Maeda, T.; et al. Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas gingivalis Lipopolysaccharide. Pharmaceuticals 2023, 16, 303. https://doi.org/10.3390/ph16020303
Tamura H, Maekawa T, Domon H, Sirisereephap K, Isono T, Hirayama S, Hiyoshi T, Sasagawa K, Takizawa F, Maeda T, et al. Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas gingivalis Lipopolysaccharide. Pharmaceuticals. 2023; 16(2):303. https://doi.org/10.3390/ph16020303
Chicago/Turabian StyleTamura, Hikaru, Tomoki Maekawa, Hisanori Domon, Kridtapat Sirisereephap, Toshihito Isono, Satoru Hirayama, Takumi Hiyoshi, Karin Sasagawa, Fumio Takizawa, Takeyasu Maeda, and et al. 2023. "Erythromycin Restores Osteoblast Differentiation and Osteogenesis Suppressed by Porphyromonas gingivalis Lipopolysaccharide" Pharmaceuticals 16, no. 2: 303. https://doi.org/10.3390/ph16020303