Eriocitrin Disrupts Erythrocyte Membrane Asymmetry through Oxidative Stress and Calcium Signaling and the Activation of Casein Kinase 1α and Rac1 GTPase
Abstract
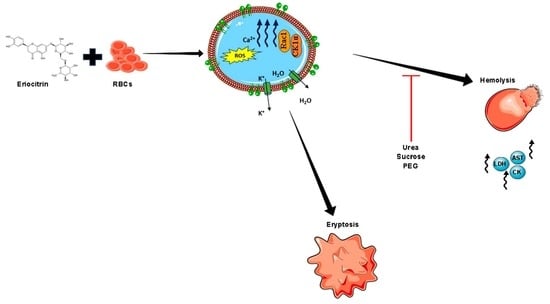
:1. Introduction
2. Results
2.1. ERN Induces Concentration-Dependent Hemolysis
2.2. ERN Increases Extracellular Acidity
2.3. ERN Stimulates Eryptosis
2.4. ERN Elevates Cytosolic Ca2+
2.5. ERN Elicits Oxidative Stress
2.6. ERN Causes Cell Shrinkage and Swelling
2.7. CK1α and Rac1 GTPase Mediate ERN-Induced Hemolysis
2.8. ERN Exhausts Intracellular Hb Stores
3. Discussion
4. Methods
4.1. Chemicals and Reagents
4.2. RBC Purification
4.3. Experimental Design
4.4. Hemolysis
4.5. Potassium
4.6. Lactate Dehydrogenase (LDH)
4.7. Aspartate Aminotransferase (AST)
4.8. Creatine Kinase (CK)
4.9. Extracellular Acidity
4.10. Acetylcholine Esterase (AChE)
4.11. Membrane Asymmetry and Cellular Morphology
4.12. Intracellular Ca2+
4.13. Oxidative Stress
4.14. Systemic Toxicity
4.15. Erythrocyte Sedimentation Rate (ESR)
4.16. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon-A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, R.; Khan, F.; Ullah, S.; Khan, A.; Al-Jahdhami, H.; Hussain, J.; Weli, A.M.; Maqsood, D.; Rahman, S.M.; Hussain, A.; et al. Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott. Antibiotics 2023, 12, 354. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef]
- Minato, K.; Miyake, Y.; Fukumoto, S.; Yamamoto, K.; Kato, Y.; Shimomura, Y.; Osawa, T. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003, 72, 1609–1616. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C.C. Eriocitrin: A review of pharmacological effects. Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Miyake, Y.; Yamamoto, K.; Okumura, K.; Taguchi, H. Apoptosis induced by the flavonoid from lemon fruit (Citrus limon BURM. f.) and its metabolites in HL-60 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1075–1078. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhou, J.; Zhang, X.; Chen, L.; Chen, K.; Huang, Z. Eriocitrin from lemon suppresses the proliferation of human hepatocellular carcinoma cells through inducing apoptosis and arresting cell cycle. Cancer Chemother. Pharmacol. 2016, 78, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chen, G.; Jing, C.; Liu, M.; Liang, B.; Gong, G.; Yu, M. Eriocitrin, a dietary flavonoid suppressed cell proliferation, induced apoptosis through modulation of JAK2/STAT3 and JNK/p38 MAPKs signaling pathway in MCF-7 cells. J. Biochem. Mol. Toxicol. 2022, 36, e22943. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lai, K.; Deng, Y.; Lu, Z.; Song, C.; Wang, W.; Xu, C.; Li, N.; Geng, Q. Eriocitrin inhibits epithelial-mesenchymal transformation (EMT) in lung adenocarcinoma cells via triggering ferroptosis. Aging 2023, 15, 10089–10104. [Google Scholar] [CrossRef]
- Visweshwar, N.; Jaglal, M.; Sokol, L.; Zuckerman, K. Chemotherapy-related anemia. Ann. Hematol. 2018, 97, 375–376. [Google Scholar] [CrossRef]
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef]
- Restivo, I.; Attanzio, A.; Tesoriere, L.; Allegra, M. Suicidal Erythrocyte Death in Metabolic Syndrome. Antioxidants 2021, 10, 154. [Google Scholar] [CrossRef]
- Lang, P.A.; Huober, J.; Bachmann, C.; Kempe, D.S.; Sobiesiak, M.; Akel, A.; Niemoeller, O.M.; Dreischer, P.; Eisele, K.; Klarl, B.A.; et al. Stimulation of erythrocyte phosphatidylserine exposure by paclitaxel. Cell Physiol. Biochem. 2006, 18, 151–164. [Google Scholar] [CrossRef]
- Mahmud, H.; Föller, M.; Lang, F. Suicidal erythrocyte death triggered by cisplatin. Toxicology 2008, 249, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Vishalakshi, G.J.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Para-tertiary butyl catechol induces eryptosis in vitro via oxidative stress and hemoglobin leakage in human erythrocytes. Toxicol. Vitr. 2018, 52, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.T. Low pH-induced hemolysis of erythrocytes is related to the entry of the acid into cytosole and oxidative stress on cellular membranes. Biochim. Biophys. Acta 1999, 1415, 349–360. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Qadri, S.M. Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purif. 2012, 33, 125–130. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Ions, the Movement of Water and the Apoptotic Volume Decrease. Front. Cell Dev. Biol. 2020, 8, 611211. [Google Scholar] [CrossRef]
- Ghashghaeinia, M.; Wesseling, M.C.; Ramos, E.; Petkova-Kirova, P.; Waibel, S.; Lang, E.; Bissinger, R.; Alzoubi, K.; Edelmann, B.; Hosseinzadeh, Z.; et al. Trifluoperazine-Induced Suicidal Erythrocyte Death and S-Nitrosylation Inhibition, Reversed by the Nitric Oxide Donor Sodium Nitroprusside. Cell Physiol. Biochem. 2017, 42, 1985–1998. [Google Scholar] [CrossRef]
- Lang, K.S.; Myssina, S.; Lang, P.A.; Tanneur, V.; Kempe, D.S.; Mack, A.F.; Huber, S.M.; Wieder, T.; Lang, F.; Duranton, C. Inhibition of erythrocyte phosphatidylserine exposure by urea and Cl. Am. J. Physiol. Renal Physiol. 2004, 286, F1046–F1053. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. Biomed. Res. Int. 2018, 2018, 9405617. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Pribush, A.; Meyerstein, D.; Meyerstein, N. Kinetics of erythrocyte swelling and membrane hole formation in hypotonic media. Biochim. Biophys. Acta 2002, 1558, 119–132. [Google Scholar] [CrossRef]
- Tkachenko, A.; Onishchenko, A. Casein kinase 1alpha mediates eryptosis: A review. Apoptosis 2023, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zelenak, C.; Eberhard, M.; Jilani, K.; Qadri, S.M.; Macek, B.; Lang, F. Protein kinase CK1alpha regulates erythrocyte survival. Cell Physiol. Biochem. 2012, 29, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, H.; Khan, A.; Yamasaki, M.; Moustaid-Moussa, N.; Al-Harrasi, A.; Rahman, S.M. Autophagy in adipogenesis: Molecular mechanisms and regulation by bioactive compounds. Biomed. Pharmacother. 2022, 155, 113715. [Google Scholar] [CrossRef] [PubMed]
- Föller, M.; Sopjani, M.; Koka, S.; Gu, S.; Mahmud, H.; Wang, K.; Floride, E.; Schleicher, E.; Schulz, E.; Münzel, T.; et al. Regulation of erythrocyte survival by AMP-activated protein kinase. FASEB J. 2009, 23, 1072–1080. [Google Scholar] [CrossRef]
- Kalfa, T.A.; Pushkaran, S.; Mohandas, N.; Hartwig, J.H.; Fowler, V.M.; Johnson, J.F.; Joiner, C.H.; Williams, D.A.; Zheng, Y. Rac GTPases regulate the morphology and deformability of the erythrocyte cytoskeleton. Blood 2006, 108, 3637–3645. [Google Scholar] [CrossRef]
- George, A.; Pushkaran, S.; Konstantinidis, D.G.; Koochaki, S.; Malik, P.; Mohandas, N.; Zheng, Y.; Joiner, C.H.; Kalfa, T.A. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 2013, 121, 2099–2107. [Google Scholar] [CrossRef]
- Ferro, E.; Goitre, L.; Retta, S.F.; Trabalzini, L. The Interplay between ROS and Ras GTPases: Physiological and Pathological Implications. J. Signal Transduct. 2012, 2012, 365769. [Google Scholar] [CrossRef]
- Attanzio, A.; Frazzitta, A.; Cilla, A.; Livrea, M.A.; Tesoriere, L.; Allegra, M. 7-Keto-Cholesterol and Cholestan-3beta, 5alpha, 6beta-Triol Induce Eryptosis through Distinct Pathways Leading to NADPH Oxidase and Nitric Oxide Synthase Activation. Cell Physiol. Biochem. 2019, 53, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Alghareeb, S.A.; Alsughayyir, J.; Alfhili, M.A. Stimulation of Hemolysis and Eryptosis by α-Mangostin through Rac1 GTPase and Oxidative Injury in Human Red Blood Cells. Molecules 2023, 28, 6495. [Google Scholar] [CrossRef] [PubMed]
- Sidoux-Walter, F.; Lucien, N.; Olives, B.; Gobin, R.; Rousselet, G.; Kamsteeg, E.J.; Ripoche, P.; Deen, P.M.; Cartron, J.P.; Bailly, P. At physiological expression levels the Kidd blood group/urea transporter protein is not a water channel. J. Biol. Chem. 1999, 274, 30228–30235. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.K.; Rehman, N.U.; Khan, F.; Ullah, O.; Halim, S.A.; Khan, A.; Anwar, M.U.; Rahman, S.M.; Csuk, R.; Al-Harrasi, A. Triazole-tethered boswellic acid derivatives against breast cancer: Synthesis, in vitro, and in-silico studies. J. Mol. Struct. 2023, 1282, 135181. [Google Scholar] [CrossRef]
- Mozaffari Nejad, A.S.; Noor, T.; Munim, Z.H.; Alikhani, M.Y.; Ghaemi, A. A bibliometric review of oncolytic virus research as a novel approach for cancer therapy. Virol. J. 2021, 18, 98. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alsughayyir, J. Metabolic exhaustion and casein kinase 1alpha drive deguelin-induced premature red blood cell death. Xenobiotica 2023, 53, 445–453. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alyousef, A.M.; Alsughayyir, J. Tamoxifen induces eryptosis through calcium accumulation and oxidative stress. Med. Oncol. 2023, 40, 333. [Google Scholar] [CrossRef]
- Zeng, B.; Huang, Y.; Chen, S.; Xu, R.; Xu, L.; Qiu, J.; Shi, F.; Liu, S.; Zha, Q.; Ouyang, D.; et al. Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis. Cell Mol. Immunol. 2022, 19, 925–943. [Google Scholar] [CrossRef]
- Ramos-Martinez, E.; Vega-Sanchez, A.E.; Perez-Rubio, G.; Mejia, M.; Buendia-Roldan, I.; Gonzalez-Perez, M.I.; Mateos-Toledo, H.N.; Andrade, W.A.; Falfan-Valencia, R.; Rojas-Serrano, J. Enhanced Activity of NLRP3 Inflammasome in the Lung of Patients with Anti-Synthetase Syndrome. Cells 2022, 12, 60. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Ueda, S.; Sakasegawa, S. Enzymatic cycling method using creatine kinase to measure creatine by real-time detection. Anal. Biochem. 2016, 506, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Altaf, S.; Muhammad, F.; Aslam, B.; Faisal, M.N. Cell membrane enveloped polymeric nanosponge for detoxification of chlorpyrifos poison: In vitro and in vivo studies. Hum. Exp. Toxicol. 2021, 40, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.; Virzi, G.M.; Marturano, D.; de Cal, M.; Marchionna, N.; Sgarabotto, L.; De Rosa, S.; Ronco, C.; Zanella, M. The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis? Int. J. Mol. Sci. 2023, 24, 14176. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.M.; Taylor, C.W. Reliable measurement of free Ca(2+) concentrations in the ER lumen using Mag-Fluo-4. Cell Calcium 2020, 87, 102188. [Google Scholar] [CrossRef]
- Mozaffari Nejad, A.S.; Fotouhi, F.; Mehrbod, P.; Keshavarz, M.; Alikhani, M.Y.; Ghaemi, A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb. Pathog. 2020, 147, 104438. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghareeb, S.A.; Alsughayyir, J.; Alfhili, M.A. Eriocitrin Disrupts Erythrocyte Membrane Asymmetry through Oxidative Stress and Calcium Signaling and the Activation of Casein Kinase 1α and Rac1 GTPase. Pharmaceuticals 2023, 16, 1681. https://doi.org/10.3390/ph16121681
Alghareeb SA, Alsughayyir J, Alfhili MA. Eriocitrin Disrupts Erythrocyte Membrane Asymmetry through Oxidative Stress and Calcium Signaling and the Activation of Casein Kinase 1α and Rac1 GTPase. Pharmaceuticals. 2023; 16(12):1681. https://doi.org/10.3390/ph16121681
Chicago/Turabian StyleAlghareeb, Sumiah A., Jawaher Alsughayyir, and Mohammad A. Alfhili. 2023. "Eriocitrin Disrupts Erythrocyte Membrane Asymmetry through Oxidative Stress and Calcium Signaling and the Activation of Casein Kinase 1α and Rac1 GTPase" Pharmaceuticals 16, no. 12: 1681. https://doi.org/10.3390/ph16121681