The Nephroprotective Effects of the Allogeneic Transplantation with Mesenchymal Stromal Cells Were Potentiated by ω3 Stimulating Up-Regulation of the PPAR-γ
Abstract
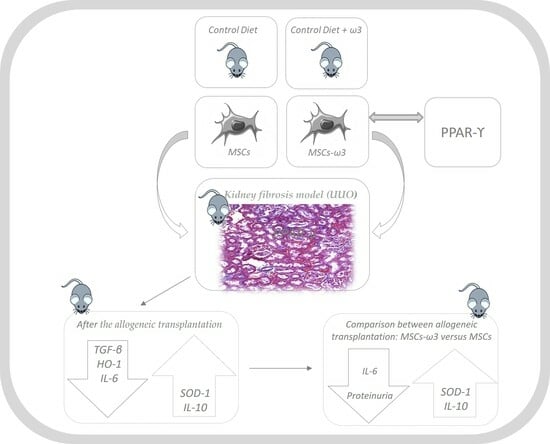
:1. Introduction
2. Results
2.1. In Vitro
2.2. In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Culture and Characterization of Bone Marrow Mesenchymal Stromal Cells (MSCs)
4.3. In Vitro Analyses of PPAR-γ
4.4. Homing of MSCs
4.5. Unilateral Ureteral Obstruction and Allogeneic Transplantation
4.6. In Vivo Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Caldas, H.C.; de Paula Couto, T.A.; Fernandes, I.M.; Baptista, M.A.; Kawasaki-Oyama, R.S.; Goloni-Bertollo, E.M.; Braile, D.M.; Abbud-Filho, M. Comparative effects of mesenchymal stem cell therapy in distinct stages of chronic renal failure. Clin. Exp. Nephrol. 2015, 19, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wu, D. Bone marrow-derived mesenchymal stem cells protect against cisplatin-induced acute kidney injury in rats by inhibiting cell apoptosis. Int. J. Mol. Med. 2013, 32, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Borges, F.T.; Convento, M.B.; Schor, N. Bone marrow-derived mesenchymal stromal cell: What next? Stem Cells Cloning Adv. Appl. 2018, 11, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Chen, J.H.; Hwang, S.M.; Wang, I.J.; Li, H.J.; Lee, R.T.; Hsieh, P.C. Salvianolic acid B-vitamin C synergy in cardiac differentiation from embryonic stem cells. Biochem. Biophys. Res. Commun. 2009, 387, 723–728. [Google Scholar] [CrossRef]
- Kang, J.X.; Wan, J.B.; He, C. Concise review: Regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem Cells 2014, 32, 1092–1098. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Zhang, H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: A meta-analysis. Clinics 2017, 72, 58–64. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Banga, A.; Unal, R.; Tripathi, P.; Pokrovskaya, I.; Owens, R.J.; Kern, P.A.; Ranganathan, G. Adiponectin translation is increased by the PPARγ agonists pioglitazone and omega-3 fatty acids. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E480–E489. [Google Scholar] [CrossRef]
- Gao, J.; Gu, Z. The Role of Peroxisome Proliferator-Activated Receptors in Kidney Diseases. Front. Pharmacol. 2022, 13, 832732. [Google Scholar] [CrossRef]
- Sharma, V.; Patial, V. Peroxisome proliferator-activated receptor gamma and its natural agonists in the treatment of kidney diseases. Front. Pharmacol. 2022, 13, 991059. [Google Scholar] [CrossRef]
- Pessoa, E.A.; Convento, M.B.; Castino, B.; Leme, A.M.; de Oliveira, A.S.; Aragão, A.; Fernandes, S.M.; Carbonel, A.; Dezoti, C.; Vattimo, M.F.; et al. Beneficial Effects of Isoflavones in the Kidney of Obese Rats Are Mediated by PPAR-Gamma Expression. Nutrients 2020, 12, 1624. [Google Scholar] [CrossRef]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef]
- Asanuma, H.; Vanderbrink, B.A.; Campbell, M.T.; Hile, K.L.; Zhang, H.; Meldrum, D.R.; Meldrum, K.K. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J. Surg. Res. 2011, 168, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, L.; Yang, Y.; Wang, Z.; Cai, Y.; Hong, T.; Chen, P. Effects of Rab7 gene up-regulation on renal fibrosis induced by unilateral ureteral obstruction. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2020, 53, e9220. [Google Scholar] [CrossRef]
- Tampe, D.; Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 226–237. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Zhen, T.; Li, J.; Xing, K.; He, L.; Zhu, S. Transcriptomic analysis of the mechanisms of alleviating renal interstitial fibrosis using the traditional Chinese medicine Kangxianling in a rat model. Sci. Rep. 2020, 10, 10682. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Shimazui, T.; Nakada, T.A.; Tateishi, Y.; Oshima, T.; Aizimu, T.; Oda, S. Association between serum levels of interleukin-6 on ICU admission and subsequent outcomes in critically ill patients with acute kidney injury. BMC Nephrol. 2019, 20, 74. [Google Scholar] [CrossRef]
- Feigerlová, E.; Battaglia-Hsu, S.F. IL-6 signaling in diabetic nephropathy: From pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev. 2017, 37, 57–65. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, R.; Xie, J.; Xiong, H.; He, J.C.; Chen, N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab. Investig. A J. Tech. Methods Pathol. 2013, 93, 801–811. [Google Scholar] [CrossRef]
- Mu, W.; Ouyang, X.; Agarwal, A.; Zhang, L.; Long, D.A.; Cruz, P.E.; Roncal, C.A.; Glushakova, O.Y.; Chiodo, V.A.; Atkinson, M.A.; et al. IL-10 suppresses chemokines, inflammation, and fibrosis in a model of chronic renal disease. J. Am. Soc. Nephrol. 2005, 16, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hong, S.; Zhu, X.; Zhang, L.; Tang, H.; Jordan, K.L.; Saadiq, I.M.; Huang, W.; Lerman, A.; Eirin, A.; et al. IL-10 partly mediates the ability of MSC-derived extracellular vesicles to attenuate myocardial damage in experimental metabolic renovascular hypertension. Front. Immunol. 2022, 13, 940093. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Lara-Villoslada, F.; Olivares, M.; Jiménez, J.; Boza, J.; Xaus, J. Il-10 expression is involved in the regulation of the immune response by omega 3 fatty acids. Nutr. Hosp. 2004, 19, 376–382. [Google Scholar] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, H.N.; Kim, H.W.; Jo, S.K.; Cho, W.Y.; Kim, H.K. IL-10 mediates rosiglitazone-induced kidney protection in cisplatin nephrotoxicity. J. Korean Med. Sci. 2010, 25, 557–563. [Google Scholar] [CrossRef]
- Hill-Kapturczak, N.; Truong, L.; Thamilselvan, V.; Visner, G.A.; Nick, H.S.; Agarwal, A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J. Biol. Biol. Chem. 2000, 275, 40904–40909. [Google Scholar] [CrossRef]
- Chuang, S.T.; Kuo, Y.H.; Su, M.J. KS370G, a caffeamide derivative, attenuates unilateral ureteral obstruction-induced renal fibrosis by the reduction of inflammation and oxidative stress in mice. Eur. J. Pharmacol. 2015, 750, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, F.X.; Zhou, H.; Lu, Y.Y.; Tan, H.; Yu, S.J.; Yuan, J.; Liu, H.; Meng, W.; Jin, Z.B. Bioenergetic Crosstalk between Mesenchymal Stem Cells and Various Ocular Cells through the Intercellular Trafficking of Mitochondria. Theranostics 2020, 10, 7260–7272. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- Lin, S.; Lin, W.; Liao, C.; Zhou, T. Nephroprotective Effect of Mesenchymal Stem Cell-Based Therapy of Kidney Disease Induced by Toxicants. Stem Cells Int. 2020, 2020, 8819757. [Google Scholar] [CrossRef]
- Fazelian, S.; Moradi, F.; Agah, S.; Hoseini, A.; Heydari, H.; Morvaridzadeh, M.; Omidi, A.; Pizarro, A.B.; Ghafouri, A.; Heshmati, J. Effect of omega-3 fatty acids supplementation on cardio-metabolic and oxidative stress parameters in patients with chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2021, 22, 160. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Mitochondria: Omega-3 in the route of mitochondrial reactive oxygen species. Int. J. Biochem. Cell Biol. 2012, 44, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Trimarchi, H.; Muryan, A.; Dicugno, M.; Young, P.; Forrester, M.; Lombi, F.; Pomeranz, V.; Iriarte, R.; Raña, M.S.; Alonso, M. Proteinuria: An ignored marker of inflammation and cardiovascular disease in chronic hemodialysis. Int. J. Nephrol. Renov. Dis. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Diretriz Brasileira para o Cuidado e a Utilização de Animais em Atividades de Ensino ou de Pesquisa Científica DBCA. Resolução Norm. MCTI. 2016. Available online: https://antigo.mctic.gov.br (accessed on 20 April 2020).
- The World Health Organisation. Diet Nutrition and the Prevention of Chronic Diseases. Report of the WHO/FAO Joint Expert Consultation, WHO, Technical Report Series 916. Available online: https://www.who.int/publications/i/item/924120916X (accessed on 24 April 2020).
- From the Joint FAP/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. Interim Summary of Conclusions and Dietary Recommendations on Total Fat & Fatt Acids, WHO, Geneva. 2008. Available online: https://www.foodpolitics.com/wp-content/uploads/FFA_summary_rec_conclusion.pdf (accessed on 24 April 2020).
- Smajilagić, A.; Aljičević, M.; Redžić, A.; Filipović, S.; Lagumdžija, A. Rat bone marrow stem cells isolation and culture as a bone formative experimental system. Bosn. J. Basic Med. Sci. 2013, 13, 27–30. [Google Scholar] [CrossRef]
- Convento, M.B.; Pessoa, E.A.; Cruz, E.; da Glória, M.A.; Schor, N.; Borges, F.T. Calcium oxalate crystals and oxalate induce an epithelial-to-mesenchymal transition in the proximal tubular epithelial cells: Contribution to oxalate kidney injury. Sci. Rep. 2017, 7, 45740. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Taussky, H.H. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J. Biol. Chem. 1954, 208, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Orsonneau, J.L.; Douet, P.; Massoubre, C.; Lustenberger, P.; Bernard, S. An improved pyrogallol red-molybdate method for determining total urinary protein. Clin. Chem. 1989, 35, 2233–2236. [Google Scholar] [CrossRef] [PubMed]





| Groups | Plasma Creatinine (mg/mL) | Plasma Urea (mg/mL) | Urinary Protein (mg/mL) | Kidney (Mean Right/g) | Kidney (Mean Left/g) |
|---|---|---|---|---|---|
| Sham | 0.54 ± 0.02 | 36.67 ± 3.87 | 1.29 ± 0.07 | 1.207 ± 0.06 | 1.192 ± 0.04 |
| Sham-MSCs | 0.64 ± 0.03 | 39.83 ± 1.72 | 0.98 ± 0.10 | 1.249 ± 0.0 | 1.216 ± 0.04 |
| Sham-MSCs-ω3 | 0.50 ± 0.01 | 40.40 ± 0.74 | 1.02 ± 0.08 | 1.110 ± 0.01 | 1.099 ± 0.02 |
| UUO | 0.61 ± 0.03 * | 45.33 ± 1.50 * | 1.89 ± 0.19 * | 1.516 ± 0.08 | 1.934 ± 0.11 # |
| UUO-MSCs | 0.62 ± 0.03 * | 45.50 ± 1.76 * | 1.36 ± 0.09 ** | 1.307 ± 0.04 | 1.853 ± 0.07 # |
| UUO-MSCs-ω3 | 0.61 ± 0.02 * | 46.00 ± 1.79 * | 0.92 ± 0.14 ** ¥ | 1.332 ± 0.03 | 2.047 ± 0.16 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.S.; Convento, M.B.; Razvickas, C.V.; Castino, B.; Leme, A.M.; da Silva Luiz, R.; da Silva, W.H.; da Glória, M.A.; Guirão, T.P.; Bondan, E.; et al. The Nephroprotective Effects of the Allogeneic Transplantation with Mesenchymal Stromal Cells Were Potentiated by ω3 Stimulating Up-Regulation of the PPAR-γ. Pharmaceuticals 2023, 16, 1484. https://doi.org/10.3390/ph16101484
de Oliveira AS, Convento MB, Razvickas CV, Castino B, Leme AM, da Silva Luiz R, da Silva WH, da Glória MA, Guirão TP, Bondan E, et al. The Nephroprotective Effects of the Allogeneic Transplantation with Mesenchymal Stromal Cells Were Potentiated by ω3 Stimulating Up-Regulation of the PPAR-γ. Pharmaceuticals. 2023; 16(10):1484. https://doi.org/10.3390/ph16101484
Chicago/Turabian Stylede Oliveira, Andreia Silva, Márcia Bastos Convento, Clara Versolato Razvickas, Bianca Castino, Ala Moana Leme, Rafael da Silva Luiz, Wesley Henrique da Silva, Maria Aparecida da Glória, Tatiana Pinotti Guirão, Eduardo Bondan, and et al. 2023. "The Nephroprotective Effects of the Allogeneic Transplantation with Mesenchymal Stromal Cells Were Potentiated by ω3 Stimulating Up-Regulation of the PPAR-γ" Pharmaceuticals 16, no. 10: 1484. https://doi.org/10.3390/ph16101484