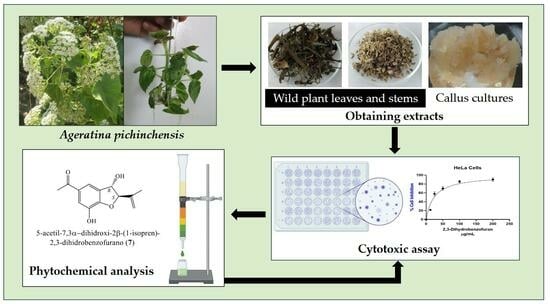
Cytotoxic Activity of Wild Plant and Callus Extracts of Ageratina pichinchensis and 2,3-Dihydrobenzofuran Isolated from a Callus Culture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxic Evaluation of Extracts
2.2. Chemical Profile of Ethyl Acetate Extract of Callus Cultures
2.3. Cytotoxic Effect of the Compound 2,3-Dihydrobenzofuran
3. Materials and Methods
3.1. Plant Material from the Wild Plants
3.2. Plant Material from Callus Cultures
3.3. Obtaining Organic Extracts
3.4. Cytotoxic Assay
3.5. Isolation of Compounds from Callus Culture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de la Medicina Tradicional Mexicana; Tomo 1–3; Instituto Nacional Indigenista: Mexico, City, Mexico, 1994; p. 1786. [Google Scholar]
- WFO. Ageratina pichinchensis (Kunth) R.M. King & H. Rob. Available online: www.worldfloraonline.org/taxon/wfo-40000000936 (accessed on 18 September 2023).
- Romero-Cerecero, O.; Rojas, G.; Navarro, V.; Herrera-Arellano, A.; Zamilpa-Alvarez, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: An explorative pilot study controlled with ketaconazole. Planta Medica 2006, 72, 1257–1261. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Román-Ramos, R.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Tortoriello, J. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J. Ethnopharmacol. 2009, 126, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortes, D.; Jiménez-Ferrer, E.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Medica 2013, 79, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Román-Ramos, R.; Tortoriello, J. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox. Planta Medica 2008, 78, 1430–1435. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Ramos-Mora, A.; Alonso-Cortés, D.; Jiménez-Ferrer, J.E.; Huerta-Reyes, M.E.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medicinal mexican plant Ageratina pichinchensis. Planta Medica 2011, 77, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Alvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a cell suspension culture of Ageratina pichinchensis (Kunth) for the improved production of anti-inflammatory compounds. Plants 2020, 9, 1398. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Bernabé-Antonio, B.; Cabañas-García, E.; Román-Guerrero, A.; Cruz-Sosa, F. Obtaining 2,3-dihydrobenzofuran and 3-epilupeol from Ageratina pichinchensis (Kunth) R. King & Ho. Rob. Cell cultures grown in shake flasks under photoperiod and darkness, and its scale-up to an airlift bioreactor for enhanced production. Molecules 2023, 28, 578. [Google Scholar]
- WHO. WHO Outlines Steps to Save 7 Million Lives from Cancer. Available online: https://www.who.int/es/news/item/04-02-2020-who-outlines-steps-to-save-7-million-lives-from-cancer (accessed on 18 September 2023).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–798. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Yao, C.-L.; Zhang, J.-Q.; Li, J.-Y.; Wei, W.-L.; Wu, S.-F.; Guo, D.-A. Traditional chinese medicine (TCM) as a source of new anticancer drugs. Nat. Prod. Rep. 2020, 9, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Obenauf, A.C. Mechanism-based combination therapies for metastatic cancer. Sci. Transl. Med. 2022, 14, eadd0887. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Gabrilovich, D.I. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immun 2011, 60, 419–423. [Google Scholar] [CrossRef]
- Sharma, H.; Parihar, L.; Parihar, P. Review on cancer and anticancer properties of some medicinal plants. J. Med. Plant Res. 2011, 5, 1818–1835. [Google Scholar]
- Howat, S.; Park, B.; Oh, I.S.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Paclitaxel: Biosynthesis, production and future prospects. N. Biotechnol. 2014, 31, 242–245. [Google Scholar] [CrossRef]
- Yan-Hua, Y.; Jia-Wang, M.; Xiao-Li, T. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar]
- Kumar, A. Vincristine and vinblastine: A review. Int. J. Med. Pharm. Sci. 2016, 6, 23–30. [Google Scholar]
- Gezici, S.; Şekeroĝlu, N. Current perspectives in the application of medicinal plants against cancer: Novel therapeutic agents. Anti-Cancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardere, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Abdalla, Y.O.A.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural products for cancer therapy: A review of their mechanism of actions and toxicity in the past decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Teiten, M.-H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2010, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.M.; Pérez, B.J.J.; Mendoza, L.M.L.; Apátiga-Castro, M.; López-Romero, J.M.; Mendoza, S.; Manzano-Ramírez, A. Antioxidants in traditional Mexicans medicine and their applications as antitumor treatments. Pharmaceuticals 2023, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 3, 761–771. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Liu, S.; Wang, Y.; Liu, G. Emerging nanomedicines of paclitaxel for cancer treatment. JCR 2022, 342, 280–294. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, S.; Ni, S.; Zhang, B.; Kung, A.C.F.; Gao, J.; Lu, A.; Zhang, G. Targeting strategies for enhancing paclitaxel specificity in chemotherapy. Front. Cell Dev. Biol. 2022, 9, 626910. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Wubulikasimu, A.; Mukhamedov, N.; Mirzaakhmedov, S.Y.; Gao, Y.; Aisa, H.; Yili, A. Biological activity of fatty acids from lipids of Orchis chusua. Chem. Nat. Compd. 2021, 57, 230–233. [Google Scholar] [CrossRef]
- Mothana, R.A.; Al-Rehaily, A.J.; Schultze, W. Chemical analysis and biological activity of the essential oils of two endemic soqotri Commiphora species. Molecules 2010, 15, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, T.; Subban, M.; Christopher, L.D.B. Structural characterization of n-hexadecanoic acid from the leaves of Ipomea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Canzoneri, M.; Bruno, M.; Rosselli, S.; Russo, A.; Cardile, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and biological activity of Salvia verbenaca essential oil. Nat. Prod. Commun. 2011, 6, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Sanseera, D.; Niwatananun, W.; Liawruangrath, B.; Liawruangrath, S.; Baramee, A.; Pyne, S.G. Chemical composition and biological activities of the essential oil from leaves of Cleidion javanicum Bl. J. Essent. Oil-Bear. Plants 2012, 15, 186–194. [Google Scholar] [CrossRef]
- Arpana, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
- Abubacker, M.N.; Deepalakshmi, T. In vitro antifungal potentials of bioactive compound methyl ester of hexadecenoic acid isolated from Annona muricata Linn. (Annonaceae) leaves. Biosci. Biotechnol. Res. Asia 2013, 10, 879–884. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Korinek, M.; El-Shazly, M.; Wetterauer, B.; El-Beshbishy, H.A.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; Wink, M.; Singab, A.N.B.; et al. Anti-allergic, anti-inflammatory, and anti-hyperglycemic activity of Chasmanthe aethiopica leaf extract and its profiling using LC/MS and GLC/MS. Plants 2021, 10, 1118. [Google Scholar] [CrossRef]
- Hua, K.-F.; Hsu, H.-Y.; Su, Y.-C.; Lin, I.-F.; Yang, S.-S.; Chen, Y.-M.; Chao, L.K. Study on the anti-inflammatory activity of methanol extract from seagrass Zostera japonica. J. Agric. Food Chem 2006, 54, 306–311. [Google Scholar] [CrossRef]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. Complementary Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Hashinaga, F.; Adbelgaleil, S.A.M.; Suganuma, T.; Akiyama, K.; Hayashi, H. Antibacterial activity of an eudesmane sesquiterpene isolated from common Varthemia, Varthemia iphionoides. J. Ethnopharmacol. 2005, 97, 237–240. [Google Scholar] [CrossRef]
- Hong, H.T.T.; Vinh, L.B.; Xuan, C.N.; Hong, Q.T. Two new eudesmane sesquiterpene glucosides from the aerial parts of Artemisia vulgaris. Nat. Prod. Res. 2023, 37, 1544–1549. [Google Scholar]
- Yang, J.-L.; Liu, L.-L.; Shi, Y.-P. Two new eudesmane sesquiterpenoids from the flowers of Chrysanthemum indicum. Nat. Prod. Bioprospect. 2019, 9, 145–148. [Google Scholar] [CrossRef]
- Prats, E.; Galindo, J.C.; Bazzalo, M.E.; León, A.; Macías, F.A.; Rubiales, D.; Jorrín. J.V. Antifungal activity of a new phenolic compound from Capitulum of a head rot-resistant sunflower genotype. J. Chem. Ecol. 2007, 33, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Salomé, M.; Pais, S.; Scheffer, J.J.C. Composition of the essential oil from suspension cultures of Achillea millefolium ssp. millefolium. Plant Cell Tissue Organ Cult. 1995, 40, 113–118. [Google Scholar] [CrossRef]
- Gutiérrez-González, J.A.; Pérez-Vázquez, A.; Torres-Colín, R.; Rangel-Grimaldo, M.; Rebollar-Ramos, D.; Mata, R. α-glucosidase inhibitors from Ageratina grandifolia. J. Nat. Prod. 2021, 84, 1573–1578. [Google Scholar] [CrossRef]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A comprehensive review. Int. J. Pharm. Sci. Res. 2011, 2, 2259–2265. [Google Scholar]
- Aboobucker, S.I.; Suza, W.P. Why do plants convert sitosterol to stigmasterol? Front. Plant Sci. 2019, 10, 354. [Google Scholar] [CrossRef]
- Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.A.; Alebiosu, C.O.; Yahaya, M.; Adamu, H.W.; Sanusi, A.; Mailafiya, M.M.; Abubaka, H. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, a38. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El, O.N.; Sheikh, R.A.; Wen, G.K.; Chiau, M.L.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Mbambo, B.; Odhav, B.; Mohanlall, V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis baker (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar]
- Navarro, A.; De las Heras, B.; Villar, A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens. Clem. Biol. Pharm. Bull. 2001, 24, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Castro, O.L.; Moreira, B.L.; de Moraes, A.M.M.; Amorim, V.; Pereira, E.; Sobrinho-Júnior, C.; Sousa de Carvalho, C.E.; da Franca, R.K.A.; Dias, R.A.D.; das Graças, L.C.A.M.; et al. In vitro effects of the neolignane 2,3-dihydrobenzofuran against Leishmania Amazonensis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Choi, M.; Jo, H.; Park, H.-J.; Sateesh, K.A.; Lee, J.; Yun, J.; Kim, Y.; Han, S.-B.; Jung, J.-K.; Cho, J.; et al. Design, synthesis, and biological evaluation of benzofuran- and 2,3-dihydrobenzofuran-2-carboxylic acid N-(substituted)phenylamide derivates as anticancer agents and inhibitors of NF-kB. Bioorganic Med. Chem. Lett. 2015, 25, 2545–2549. [Google Scholar] [CrossRef]
- Di Micco, S.; Spatafora, C.; Cardullo, N.; Riccio, R.; Fischer, K.; Pergola, C.; Koeberle, A.; Werz, O.; Chalal, M.; Vervandier-Fasseur, D.; et al. 2,3-dihydrobenzofuran privileged structures as a new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem. 2016, 24, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.R.; Lopes, L.M.X. 2,3-dihydrobenzofuran neolignans from Aristolochia pubescens. Phytochemistry 1999, 52, 345–350. [Google Scholar] [CrossRef]
- Farooq, U.; Naz, S.; Shams, A.; Raza, Y.; Ahmed, A.; Rashid, U.; Sadiq, A. Isolation of dihydrobenzofuran derivates from ethnomedicinal species Polygonum barbatum as anticancer compounds. Biol. Res. 2019, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Ran, X.-K.; Riaz, M.; Yu, M.; Cai, Q.; Dou, D.-Q.; Metwaly, A.M.; Kang, T.-G.; Cai, D.-C. Chemical constituents of stems and leaves of Tagetespatula L. and its fingerprint. Molecules 2019, 24, 3911. [Google Scholar] [CrossRef]
- Askari, V.R.; Fereydouni, N.; Rahimi, V.B.; Askari, N.; Sahebkar, A.H.; Rahmanian-Devin, P.; Samzadeh-Kermani, A. β-amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ1/Mφ2 balances. Biomed. Pharmacother. 2018, 101, 438–446. [Google Scholar] [CrossRef]
- Krishnan, K.; Mathew, L.E.; Vijayalakshmi, N.R.; Helen, A. Anti-inflammatory potential of β-amyrin, a triterpenoid isolated from Costus igneus. Inflammopharmacology 2014, 22, 373–385. [Google Scholar] [CrossRef]
- Shan, L.Y.; Chuan, T.T.; Siow, P.T.; Awang, K.; Mohd, N.H.; Azlan, N.M.; Kartini, A. Cytotoxic, antibacterial and antioxidant activity of triterpenoids from Kopsia singapurensis Ridl. J. Chem. Pharm. Res. 2014, 6, 815–822. [Google Scholar]
- Mishra, T.; Arya, R.K.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.K.; Rana, T.S.; Datta, D. Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. PLoS ONE 2016, 11, e0159430. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.A.H.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Anti-inflammatory effect of α, β-amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model acute periodontitis. Inflammopharmacology 2008, 16, 48–52. [Google Scholar] [CrossRef]
- Lima, E.M.; Nascimiento, A.M.; Lenz, D.; Scherer, R.; Meyrelles, S.S.; Boëchat, A.P.; Andrade, T.U.; Endringer, D.C. Triterpenes from the Protium heptaphyllum resin-chemical composition and cytotoxicity. Rev. Bras. Farmacogn. 2014, 24, 399–407. [Google Scholar] [CrossRef]
- Da Silva, T.B.C.; Costa, C.O.D.; Galvão, A.F.C.; Bomfim, L.M.; Rodrigues, A.C.B.C.; Mota, M.C.S.; Dantas, A.A.; dos Santos, T.R.; Soares, M.B.P.; Bezerra, D.P. Cytotoxic potential of selected medicinal plants in northeast Brazil. Complement Altern. Med. 2016, 16, 199. [Google Scholar] [CrossRef]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-amyrin and Alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profund anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef]
- Melo, C.M.; Morais, T.C.; Tomé, A.R.; Brito, G.A.C.; Chaves, M.H.; Rao, V.S.; Santos, F.A. Anti-inflammatory effect of α, β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflamm. Res. 2011, 60, 673–681. [Google Scholar] [CrossRef]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragão, G.F. Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: A literature review. Fundam. Clin. Pharmacol. 2019, 33, 4–12. [Google Scholar] [CrossRef]
- Ambati, G.G.; Yadav, K.; Maurya, R.; Kondepudi, K.K.; Bishnoi, M.; Jachak, S.M. Evaluation of the in vitro and in vivo anti-inflammatory activity of Gymnosporia montana (Roth). Benth leaves. J. Ethnopharmacol. 2022, 297, 115539. [Google Scholar] [CrossRef]
- Cardoso, B.K.; de Olivera, H.L.; Melo, U.Z.; Mariano, F.C.M.; de Araujo, A.C.C.F.; Gonçalves, J.E.; Laverde, A., Jr.; Barion, R.M.; Linde, G.A.; Cristiani, G.Z. Antioxidant activity of α, and β-amyrin isolated from Myrcianthes pungens leaves. Nat. Prod. Res. 2018, 12, 1777–1781. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-amyrin and β-amyrin isolated from Celastrus hindsii leaves and their antioxidant, anti-xanthine oxidase, and anti-tyrosinase potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef] [PubMed]
- Akramov, D.K.; Mamadalieva, N.Z.; Porzel, A.; Hussain, H.; Dube, M.; Akhmedov, A.; Altyar, A.E.; Ashour, M.L.; Wessjohann, L.A. Sugar containing compounds and biological activities of Lagochilus setulosus. Molecules 2021, 6, 1755. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxic and apoptosis studies of B-sitosteol-3-O-glucoside and B-amyrin from Prunus africana. Afr. J. Tradit. Complement Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.F.; Ibrahim, A.K.; Yamada, K.; Ahmed, S.A. Cytotoxic and anti-inflammatory compounds from red sea grass Thalassodendron ciliatum. Med. Chem. Res. 2018, 27, 1238–1244. [Google Scholar] [CrossRef]
- Oyetunde-Joshua, F.; Moodley, R.; Cheniah, H.; Khan, R. Phytochemical and Biological studies of Helichrysum acutatum DC. Multifaceted J. Field Nat. Prod. Pharmacogn. 2022, 14, 603–609. [Google Scholar] [CrossRef]
- Rashed, K.N.; Ćiriĉ, A.; Glamoĉlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sokoviĉ, M. Identification of the bioactive constituents and the antibacterial, antifungal and cytotoxic activities of different fractions from Cestrum nocturnum L. Jordan J. Biol. Sci. 2018, 11, 273–279. [Google Scholar]
- Ezzat, M.I.; Ezzat, S.M.; El, D.K.S.; El, F.A.M. In vitro evaluation of cytotoxic activity of the ethanol extract and isolated compounds from the corms of Liatris spicata (L.) wild on HepG2. Nat. Prod. Res. 2017, 11, 1325–1328. [Google Scholar] [CrossRef]
- Corrêa, G.M.; Abreu, V.G.D.C.; Martins, D.A.D.A.; Aparecida, T.J.A.; Fontoura, H.D.S.; Carmona, C.D.; Piló-Veloso, D.; Alcântara, A.F.D.C. Anti-inflammatory and antimicrobial activities of steroids and triterpenes isolates from aerial parts of Justicia acumintissima (Acanthaceae). Int. J. Pharm. Pharm. Sci. 2014, 6, 75–81. [Google Scholar]
- Yuan, L.; Shen, M.; Jianhua, X.S.J. Phytosterols suppress phagocytosis and inhibit inflammatory mediators responses in RAW 264.7 macrophages and the correlation with their structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef]
- Hassan, E.M.; Matloub, A.A.; Aboutabl, M.E.; Ibrahim, N.A.; Mohamed, S.M. Assessment of anti-inflammatory, antinociceptive, immunomodulatory, and antioxidant activities of Cajanus cajan L. seeds cultivated in Egypt and its phytochemical composition. Pharm. Biol. 2016, 8, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Kargutkar, S.; Brijesh, S. Anti-inflammatory evaluation and characterization of leaf extract of Ananas comosus. Inflammopharmacology 2018, 26, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J. Physiol. Pharmacol. 2014, 2, 309–315. [Google Scholar]
- Koshak, A.E.; Adballah, H.M.; Esmat, A.; Rateb, M.E. Anti-inflammatory activity and chemical characterization of Opuntia ficus-indica seed oil cultivated in Saudi Arabia. Arab. J. Sci. Eng. 2020, 45, 4571–4578. [Google Scholar] [CrossRef]
- Duan, J.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A new cytotoxic prenylated dihydrobenzofuran derivate and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef]
- Nousheen, A.; Chandrakanth, M.; Karuna, S.B.; Laxmi, S.V. Novel diastereoselective trans 2,3-dihydrobenzofuran derivates: Tandem synthesis, crystal structure, antioxidant and anticancer activity. J. Mol. Struct. 2022, 1261, 132899. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Bork, P.M.; Lienhard, M.S.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef]
- Nemati, F.; Ali, A.D.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran. Red Crescent Med. J. 2013, 15, e8871. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Varalakshmi, K.N. Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J. Nat. Pharm. 2011, 2, 138–142. [Google Scholar]
- Khodarahmi, G.A.; Ghasemi, N.; Hassanzadeh, F.; Safaie, M. Cytotoxic effects of different extract and latex of Ficus Carica L. on HeLa cell line. Iran. J. Pharm. Res. IJPR 2011, 10, 273–277. [Google Scholar]
- Li, Z.-B.; Wang, J.-Y.; Jiang, B.; Zhang, X.-L.; An, L.-J.; Bao, Y.-M. Benzobijuglone, a novel cytotoxic compound from Juglans mandshurica, induced apoptosis in HeLa cervical cancer cells. Phytomedicine 2007, 14, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Gopiesh, K.V.; Kannabiran, K. Anticancer-cytotoxic activity of saponins isolated from the leaves of Gymnema sylvestre and Eclipta prostrata on HeLa cells. Int. J. Green Pharm. 2009, 3, 227–229. [Google Scholar]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of Ageratina pichinchensis extract in patients with vulvovaginal candidiasis. A randomized, double-blind, and controlled pilot study. Phytother. Res. 2017, 31, 885–890. [Google Scholar] [CrossRef] [PubMed]





| Cellular Line | Wild Plant Leaves | Wild Plant Stems | Callus Cultures | Paclitaxel * (µg/mL) | |||
|---|---|---|---|---|---|---|---|
| EAE (µg/mL) | ME (µg/mL) | EAE (µg/mL) | ME (µg/mL) | EAE (µg/mL) | ME (µg/mL) | ||
| HeLa | 161.49 ± 4.91 | >200 | >200 | >200 | 94.79 ± 2.09 | 150.9 ± 7.5 | 0.017 ± 1.03 × 10−3 |
| PC-3 | 188.66 ± 10.42 | >200 | >200 | >200 | 121.21 ± 9.25 | 168.6 ± 4.5 | 0.013 ± 1.96 × 10−3 |
| Huh-7 | >200 | >200 | >200 | >200 | 132.80 ± 8.51 | >200 | 0.021 ± 2.73 × 10−3 |
| HepG2 | >200 | >200 | >200 | >200 | 122.97 ± 2.54 | >200 | 8.57 × 10−3 ± 1.28 × 10−3 |
| MCF-7 | >200 | >200 | >200 | >200 | 191.30 ± 6.41 | >200 | 0.018 ± 3.5 × 10−3 |
| HaCat | >200 | >200 | >200 | >200 | >200 | >200 | 0.097 ± 12.80 × 10−3 |
| Cell Cancer Line | IC50 (µg/mL) |
|---|---|
| HeLa | 23.86 ± 2.5 |
| PC-3 | 80.36 ± 4.63 |
| Huh-7 | 79.98 ± 3.78 |
| HepG2 | 74.62 ± 2.02 |
| MCF-7 | 107.20 ± 1.52 |
| HaCat | 123.50 ± 15.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Encarnación-García, J.G.; Marquina-Bahena, S.; Sánchez-Carranza, J.N.; Bernabé-Antonio, A.; Domínguez-Villegas, V.; Cabañas-García, E.; Cruz-Sosa, F. Cytotoxic Activity of Wild Plant and Callus Extracts of Ageratina pichinchensis and 2,3-Dihydrobenzofuran Isolated from a Callus Culture. Pharmaceuticals 2023, 16, 1400. https://doi.org/10.3390/ph16101400
Sánchez-Ramos M, Encarnación-García JG, Marquina-Bahena S, Sánchez-Carranza JN, Bernabé-Antonio A, Domínguez-Villegas V, Cabañas-García E, Cruz-Sosa F. Cytotoxic Activity of Wild Plant and Callus Extracts of Ageratina pichinchensis and 2,3-Dihydrobenzofuran Isolated from a Callus Culture. Pharmaceuticals. 2023; 16(10):1400. https://doi.org/10.3390/ph16101400
Chicago/Turabian StyleSánchez-Ramos, Mariana, José Guillermo Encarnación-García, Silvia Marquina-Bahena, Jessica Nayelli Sánchez-Carranza, Antonio Bernabé-Antonio, Valeri Domínguez-Villegas, Emmanuel Cabañas-García, and Francisco Cruz-Sosa. 2023. "Cytotoxic Activity of Wild Plant and Callus Extracts of Ageratina pichinchensis and 2,3-Dihydrobenzofuran Isolated from a Callus Culture" Pharmaceuticals 16, no. 10: 1400. https://doi.org/10.3390/ph16101400