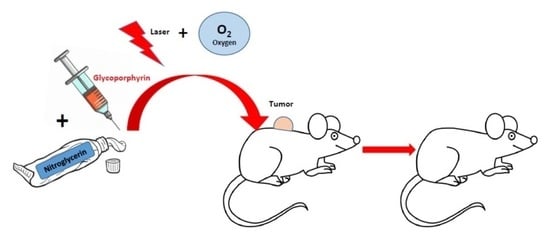
Increased PDT Efficacy When Associated with Nitroglycerin: A Study on Retinoblastoma Xenografted on Mice
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Nitroglycerin, PS and Treatment Protocols
4.2. Laser Illumination
4.3. MRI
4.4. Tumor Model and Animal Handling
4.5. Histology
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Yoshida, M.; Oishi, A.; Miyake, M.; Ooto, S.; Tamura, H.; Miyata, M.; Takahashia, A.; Hata, M.; Yamashiro, K.; Tsujikawa, A. Rescue photodynamic therapy for age-related macular degeneration refractory to anti-vascular endothelial growth factor monotherapy. Photodiagn. Photodyn. Ther. 2022, 38, 102745. [Google Scholar] [CrossRef]
- Kohoutova, D.; Haidry, R.; Banks, M.; Butt, M.A.; Dunn, J.; Thorpe, S.; Lovat, L. Long-term outcomes of the randomized controlled trial comparing 5-aminolaevulinic acid and Photofrin photodynamic therapy for Barrett’s oesophagus related neoplasia. Scand. J. Gastroenterol. 2018, 53, 527–532. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.; Zhang, L.; Zhang, Y.; Yan, G.; Zhang, H.; Liu, X.; Yang, J.; Wang, P.; Zhang, G.; et al. A prospective study of adverse reactions of ALA-PDT for acne vulgaris. Photodiagn. Photodyn. Ther. 2022, 38, 102752. [Google Scholar] [CrossRef]
- Shen, S.; Feng, J.; Song, X.; Xiang, W. Efficacy of photodynamic therapy for warts induced by human papilloma virus infection: A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2022, 39, 102913. [Google Scholar] [CrossRef]
- Assikar, S.; Labrunie, A.; Kerob, D.; Couraud, A.; Bedane, C. Daylight photodynamic therapy with methyl amino levulinate cream is as effective as conventional photodynamic therapy with blue light in the treatment of actinic keratosis: A controlled randomized intra-individual study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1730–1735. [Google Scholar] [CrossRef]
- Ballut, S.; Makky, A.; Chauvin, B.; Michel, J.P.; Kasselouri, A.; Maillard, P.; Rosilio, V. Tumor targeting in photodynamic therapy. From glycoconjugated photosensitizers to glycodendrimeric one. Concept, design and properties. Org. Biomol. Chem. 2012, 10, 4485–4495. [Google Scholar] [CrossRef]
- Wang, P.; Suna, S.; Mab, H.; Sunb, S.; Zhaob, D.; Wanga, S.; Lianga, X. Treating tumors with minimally invasive therapy: A review. Mater. Sci. Eng. C 2020, 108, 110198. [Google Scholar] [CrossRef] [PubMed]
- Poyer, F.; Thomas, C.D.; Garcia, G.; Croisy, A.; Carrez, D.; Maillard, P.; Lupu, M.; Mispelter, J. PDT induced bystander effect on human xenografted colorectal tumors as evidence by sodium MRI. Photodiagn. Photodyn. Ther. 2012, 9, 303–309. [Google Scholar] [CrossRef]
- Lupu, M.; Thomas, C.D.; Maillard, P.; Loock, B.; Chauvin, B.; Aerts, I.; Croisy, A.; Belloir, E.; Volk, A.; Mispelter, J. 23Na MRI longitudinal follow-up of PDT in a xenograft model of human retinoblastoma. Photodiagn. Photodyn. Ther. 2009, 6, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Poyer, F.; Maillard, P.; Chauvin, B.; Lupu, M.; Mispelter, J. Cellular density, a major factor involved in PDT cytotoxic responses. Study on three different lines of human retinoblastoma grafted on nude mice. Photodiagn. Photodyn. Ther. 2015, 12, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Cui, W.; Gong, H.; Liang, C.; Shi, X.; Li, Z.; Sun, B.; Liu, Z. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale 2014, 6, 11219–11225. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.K.; Wykoff, C.C.; Berrocal, A.M.; Hess, D.J.; Murray, T.G. Lasers for the treatment of intraocular tumors. Lasers Med. Sci. 2013, 28, 1025–1034. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Shen, Z.; Ma, Q.; Zhou, X.; Zhang, G.; Hao, G.; Sun, Y.; Cao, J. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater. 2021, 13, 39. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qui, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive levels and tumor growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Fujuhara, M.; Sakai, H. Biocompatibility of HbV: Liposome encapsulated haemoglobin molecules-liposome effects on immune function. J. Funct. Biomater. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.-H.; Yang, G.; Fang, F. Hyperbaric Oxygen Combined with 5-Aminolevulinic Acid Photodynamic Therapy Inhibited Human Squamous Cell Proliferation. Biol. Pharm. Bull. 2019, 42, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Luo, Z.; Chen, Y.; Yeo, J.C.C.; Li, Z.; Wu, Y.-L.; He, C. Oxygen self-supplied enzyme nanogels for tumor targeting with amplified synergistic starvation and photodynamic therapy. Acta Biomater. 2022, 142, 274–283. [Google Scholar] [CrossRef]
- Vlay, S.C.; Cohn, P.F. Nitrate therapy in angina and congestive heart failure. Cardiology 1985, 72, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Fang, J.; Maeda, H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009, 100, 2426–2430. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Qiao, B.; Luo, Y.; Cao, J.; Fan, K.; Hu, X.; Hao, L.; Cao, Y.; Zhang, Q.; Wang., Z. Increased photodynamic therapy sensitization in tumors using a nitric oxide-based nanoplatform with ATP production blocking capability. Theranostics 2021, 11, 1953–1969. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: Novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide 2008, 19, 205–216. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Maeda, H.; Noguchi, Y.; Sato, K.; Akaike, T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn. J. Cancer Res. 1994, 85, 331–334. [Google Scholar] [CrossRef]
- Yasuda, H.; Nakayama, K.; Watanabe, M.; Suzuki, S.; Fuji, H.; Okinaga, S.; Kanda, A.; Zayasu, K.; Sasaki, T.; Asada, M.; et al. Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin. Cancer Res. 2006, 12, 6748–6757. [Google Scholar] [CrossRef] [Green Version]
- Rapozzi, V.; della Pietra, E.; Zorzet, S.; Zacchigna, M.; Bonavida, B.; Xodo, L.E. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide 2013, 30, 26–35. [Google Scholar] [CrossRef]
- Schündeln, M.M.; Hauffa, P.K.; Bauer, J.J.; Temming, P.; Sauerwein, W.; Biewald, E.; Bornfeld, N.; Hauffa, B.P.; Grasemann, C. Pediatric survivors of retinoblastoma are at risk for altered bone metabolism after chemotherapy treatment early in life. Pediatr. Hematol. Oncol. 2015, 32, 455–466. [Google Scholar] [CrossRef]
- Aggarwal, H.; Kumar, P. Late effects of treatment in survivors of retinoblastoma in India: Are we on the road to recovery? S. Asian J. Cancer 2016, 5, 22–36. [Google Scholar] [CrossRef]
- Temming, P.; Arendt, M.; Viehmann, A.; Eisele, L.; Le Guin, C.H.; Schündeln, M.M.; Biewald, E.; Mäusert, J.; Wieland, R.; Bornfeld, N.; et al. How Eye-Preserving Therapy Affects Long-Term Overall Survival in heritable retinoblastoma survivors. J. Clin. Oncol. 2016, 34, 3183–3188. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lupu, M.; Walczak, C.; Volk, A.; Mispelter, J. Cisplatin treatment Monitoring by Sodium MRI Relaxometry at 4.7T in colorectal tumors implanted on mice. Proc. Intl. Soc. Magn. Reson. Med. 2008, 16, 2795. [Google Scholar]
- Bruschi, M.L.; da Silva, J.B.; Rosseto, H.C. Photodynamic therapy of psoriasis using photosensitizers of vegetable origin. Curr. Pharm. Des. 2019, 25, 2279–2291. [Google Scholar] [CrossRef]
- Ramirez, D.P.; Moriyama, L.T.; de Oliveira, E.R.; Inada, N.M.; Bagnato, V.S.; Kurachi, C.; Salvio, A.G. Single visit PDT for basal cell carcinoma—A new therapeutic protocol. Photodiagn. Photodyn. Ther. 2019, 26, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.S.; Lilge, L.; Nesbitt, M.; Dumoulin-White, R.J.; Mandel, A.; Jewett, M.A.S. A phase 1b clinical study of intravesical photodynamic therapy in patients with bacillus almette-Guérin–unresponsive non–muscle-invasive bladder cancer. Eur. Urol. 2022, 41, 105–111. [Google Scholar] [CrossRef]
- Usuda, J.; Inoue, T.; Tsuchida, T.; Ohtani, K.; Maehara, S.; Ikeda, N.; Ohsaki, Y.; Sasaki, T.; Oka, K. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagn. Photodyn. Ther. 2020, 30, 101698. [Google Scholar] [CrossRef] [PubMed]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An updated systematic review on focal therapy in localized prostate cancer: What has changed over the past 5 years? Eur. Urol. 2022, 88, 3–53. [Google Scholar] [CrossRef] [PubMed]
- Ferreira dos Santos, A.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy-current limitations and novel approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Ibbotson, S.H.; Wong, T.H.; Morton, C.A.; Collier, N.J.; Haylett, A.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Rhodes, L.E.; Seukeran, D.C.; et al. Management of adverse effects of topical PDT. Br. J. Dermatol. 2019, 180, 715–729. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent advances in strategies for overcoming hypoxia in photodynamic therapy of cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hu, N.; Yang, S.; Yang, Z.; Hu, L.; Wang, X.; Wen, N. Nimotuzumab shows an additive effect to inhibit cell growth of ALA-PDT treated oral cancer cells. Photodiagn. Photodyn. Ther. 2022, 38, 102817. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Vignion-Dewalle, A.S.; Thecua, E.; Lecomte, F.; Maire, C.; Deleporte, P.; Béhal, H.; Kerob, D.; Duhamel, A.; Mordon, S.; et al. Photodynamic therapy for actinic keratosis of the forehead and scalp: A randomized, controlled, phase II clinical study evaluating the noninferiority of a new protocol involving irradiation with a light-emitting, fabric-based device (the Flexitheralight protocol) compared with the conventional protocol involving irradiation with the Aktilite CL 128 lamp. Br. J. Dermatol. 2019, 180, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Buzinari, T.C.; de Moraes, T.F.; Conceição-Filho, J.C.; Cárnio, E.C.; Almeida-Lopes, L.; Salgado, H.C.; Rodrigues, G.J. Nitric oxide storage levels modulate vasodilation and the hypotensive effect induced by photobiomodulation using an aluminum gallium arsenide (AlGaAs) diode laser (660 nm). Lasers Med. Sci. 2022, 37, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Ridnour, L.A.; McGinity, C.L.; Bhattacharyya, D.; Wink, D.A. Nitric Oxide and Cancer: When to Give and When to Take Away? Inorg. Chem. 2021, 60, 15941–15947. [Google Scholar] [CrossRef] [PubMed]
- Lohr, N.L.; Keszler, A.; Pratt, P.; Bienengraber, M.; Warltier, D.C.; Hogg, N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J. Mol. Cell. Cardiol. 2009, 47, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Divakaran, S.; Loscalzo, J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef]
- Rapozzi, V.; della Pietra, E.; Bonavida, B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015, 6, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Girotti, A.W. Nitric Oxide-elicited Resistance to Antitumor Photodynamic Therapy via Inhibition of Membrane Free Radical-mediated Lipid Peroxidation. Photochem. Photobiol. 2021, 97, 653–663. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer. Ther. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Nagai, H.; Yasuda, H.; Hatachi, Y.; Xue, D.; Sasaki, T.; Yamaya, M.; Sakamori, Y.; Togashi, Y.; Masago, K.; Ito, I.; et al. Nitric oxide (NO) enhances pemetrexed cytotoxicity via NO-cGMP signaling in lung adenocarcinoma cells in vitro and in vivo. Int. J. Oncol. 2012, 41, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 53–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laville, I.; Pigaglio, S.; Blais, J.-C.; Doz, F.; Loock, B.; Maillard, P.; Grierson, D.S.; Blais, J. Photodynamic efficiency of diethylene glycol-linked glycoconjugated porphyrins in human retinoblastoma cells. J. Med. Chem. 2006, 49, 2558–2567. [Google Scholar] [CrossRef]
- Zhu, T.C.; Liu, B.; Kim, M.M.; McMillan, D.; Liang, X.; Finlay, J.C.; Busch, T.M. Comparison of singlet oxygen threshold dose for PDT. Proc. SPIE Int. Soc. Opt. Eng. 2014, 8931. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.H.; Murant, R.S.; Bryant, R.G.; Knox, R.S.; Gibson, S.L.; Hilf, R. Oxygen consumption and diffusion effects in photodynamic. Ther. Radiat. Res. 1991, 126, 296–303. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, C.D.; Lupu, M.; Poyer, F.; Maillard, P.; Mispelter, J. Increased PDT Efficacy When Associated with Nitroglycerin: A Study on Retinoblastoma Xenografted on Mice. Pharmaceuticals 2022, 15, 985. https://doi.org/10.3390/ph15080985
Thomas CD, Lupu M, Poyer F, Maillard P, Mispelter J. Increased PDT Efficacy When Associated with Nitroglycerin: A Study on Retinoblastoma Xenografted on Mice. Pharmaceuticals. 2022; 15(8):985. https://doi.org/10.3390/ph15080985
Chicago/Turabian StyleThomas, Carole D., Mihaela Lupu, Florent Poyer, Philippe Maillard, and Joël Mispelter. 2022. "Increased PDT Efficacy When Associated with Nitroglycerin: A Study on Retinoblastoma Xenografted on Mice" Pharmaceuticals 15, no. 8: 985. https://doi.org/10.3390/ph15080985