Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction

2. Results
2.1. Patients
Demographic and Baseline Characteristics
2.2. Efficacy
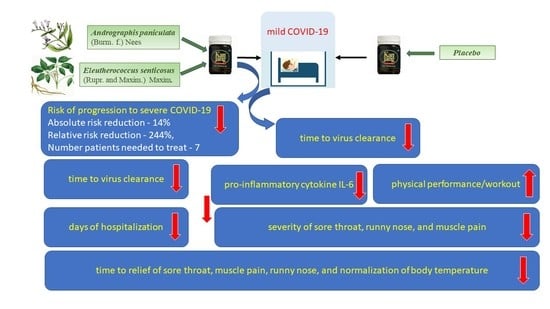
2.2.1. Primary Endpoints
The Rate of Patients with Clinical Deterioration and Duration of Hospitalization in the Treatment Group and Control Group
- Group A’s (Kan Jang, 30 patients) disease progression rate (10.0%).
- Group B’s (placebo, 41 patients) disease progression rate (24.39%).
Virus Clearance and Body Temperature
The Duration and Severity of Inflammatory Symptoms
2.2.2. Secondary Endpoints
Blood Serum Markers of Immune Response and Inflammation
Physical Activity, Physical and Cognitive Performance, and Quality of Life Scores
2.3. Safety
3. Discussion
4. Materials and Methods
4.1. Study Design, Recruitment, and Screening of Patients, Schedule of Examinations
4.1.1. Study Population, Inclusion, and Exclusion Criteria
4.1.2. Participant Withdrawal
4.1.3. Data Sets Analyzed
4.2. Intervention and Comparator
4.2.1. Doses and Treatment Regimens
4.2.2. Randomization and Blinding
4.2.3. Allocation Concealment
4.2.4. Implementation and Blinding
4.2.5. Evaluation of Compliance
4.3. Efficacy and Safety Outcomes and Endpoints
4.4. Statistical Analysis
Sample Size Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ClinicalTrials.Gov. Long Covid. Available online: https://clinicaltrials.gov/ct2/results?cond=mild+COVID&age_v=&gndr=&type=Intr&rslt=&Search=Apply (accessed on 30 May 2022).
- Wanaratna, K.; Leethong, P.; Inchai, N.; Chueawiang, W.; Sriraksa, P.; Tabmee, A.; Sirinavin, S. Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial. medRxiv 2021, 1–19. Available online: https://www.semanticscholar.org/paper/Efficacy-and-safety-of-Andrographis-paniculata-in-A-Wanaratna-Leethong/1f40a6181de4e0f15ab296673794210e82236455 (accessed on 12 August 2022).
- Rattanaraksa, D.; Khempetch, R.; Poolwiwatchaikool, U.; Nimitvilai, S.; Loatrakul, O.; Srimanee, P. The efficacy and safety of Andrographis paniculata extract for treatment of COVID-19 patients with mild symptoms. Reg. Med. J. 2022, 40, 269–281. [Google Scholar]
- Tanwettiyanont, J.; Piriyachananusorn, N.; Sangsoi, L.; Boonsong, B.; Sunpapoa, C.; Tanamatayarat, P.; Kanchanasurakit, S.; Na-Ek, N. The efficacy of Andrographis paniculata (Burm.f.) Wall. ex Nees crude extract in hospitalized mild COVID-19 patients: A retrospective cohort study. medRxiv 2022. [Google Scholar]
- Identifier TCTR20210514003—Andrographolide as a Medical Tool for Reduction of Hospitalization in Mild or Asymptomatic COVID-19 Patients: A Randomized Double-Blind Placebo Controlled Trial. TCTR; 14/05/2021; TrialID: TCTR20210514003. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-TCTR20210514003 (accessed on 12 August 2022).
- Identifier TCTR20210809004—Comparison Efficacy and Safety of Andrographis paniculata Extract Capsules and Placebo in COVID-19 Patients: Double Blind Randomized Control Trial. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-TCTR20210809004 (accessed on 12 August 2022).
- Identifier TCTR20210906002—Prospective Study of Andrographolide and Favipiravir versus Favipiravir Monotherapy to Prevent Severe Pulmonary Involvement in Patients with COVID-19. Available online: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/ictrp-TCTR20210906002 (accessed on 12 August 2022).
- Andrographis paniculata vs. Boesenbergia rotunda vs. Control in Asymptomatic COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05019326?term=Andrographis&cond=COVID-19&draw=2&rank=1 (accessed on 30 May 2022).
- ClinicalTrials.Gov. Efficacy Evaluation of Shen Cao Gan Jiang Tang on Mild and Moderate COVID-19 Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT05055427 (accessed on 16 February 2022).
- Effect of Bronchipret on Antiviral Immune Response in Patients with Mild COVID-19-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05276375?term=Bronchipret&type=Intr&cond=mild+COVID&draw=2&rank=1 (accessed on 30 May 2022).
- Hu, K.; Guan, W.J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 2021, 85, 153242. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov Identifier: NCT04467918 CANnabiDiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE). Available online: https://clinicaltrials.gov/ct2/show/NCT04467918?term=Cannabidiol&type=Intr&cond=mild+COVID&draw=2&rank=1 (accessed on 30 May 2022).
- ClinicalTrials.Gov Identifier: NCT04387240. Evaluating the Efficacy of Artesunate in Adults with Mild Symptoms of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04387240?term=Artemisinin+%2F+Artesunate&type=Intr&cond=mild+COVID&draw=2&rank=1 (accessed on 30 May 2022).
- ClinicalTrials.Gov Identifier: NCT04400890. Randomized Proof-of-Concept Trial to Evaluate the Safety and Explore the Effectiveness of Resveratrol, a Plant Polyphenol, for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04400890?term=Resveratrol&type=Intr&cond=mild+COVID&draw=2&rank=1 (accessed on 30 May 2022).
- ClinicalTrials.Gov Identifier: NCT04603690. Oral Curcumin, Quercetin and Vitamin D3 Supplements for Mild to Moderate Symptoms of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04603690?term=Curcumin&type=Intr&cond=mild+COVID&draw=2&rank=2 (accessed on 30 May 2022).
- ClinicalTrials.Gov Identifier: NCT05130671. Nutritional Supplementation of Flavonoids Quercetin and Curcumin for Early Mild Symptoms of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05130671?term=Curcumin&type=Intr&cond=mild+COVID&draw=2&rank=1 (accessed on 30 May 2022).
- Zhang, X.; Lv, L.; Zhou, Y.; Xie, L.; Xu, Q.; Zou, X.; Ding, Y.; Tian, J.; Fan, J.; Fan, H.; et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 2021, 35, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
- Karosanidze, I.; Kiladze, U.; Kirtadze, N.; Giorgadze, M.; Amashukeli, N.; Parulava, N.; Iluridze, N.; Kikabidze, N.; Gudavadze, N.; Gelashvili, L.; et al. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15, 345. [Google Scholar] [CrossRef]
- Yuvejwattana, S. Thailand Clears Use of Herbal Medicine for COVID-19 Treatment. Bloomberg. Available online: https://www.bloomberg.com/news/articles/2020-12-30/thailand-clears-use-of-herbal-medicine-for-covid-19-treatment (accessed on 30 May 2022).
- Yearsley, C. Thailand Approves Asian Herb Andrographis to Treat COVID-19. HerbalGram 2021, 129, 35–37. [Google Scholar]
- Karbwang, J.; Na-Bangchang, K. Repurposed drugs for COVID-19 treatment. J. Thai Trad. Alt. Med. 2021, 19, 285–302. [Google Scholar]
- Protasova, S.F.; Zykov, M.P. Antiviral effect of Eleutherococcus in experimental influenza infection. In Proceedings of the 2nd International Symposium on Eleutherococcus, New Data on Eleutherococcus, Moscow, Russia, 1984; pp. 170–174. [Google Scholar]
- Glatthaar-Saalmüller, B.; Sacher, F.; Esperester, A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus. Antivir. Res. 2001, 50, 223–228. [Google Scholar] [CrossRef]
- Yan, W.; Chen, J.; Wei, Z.; Wang, X.; Zeng, Z.; Tembo, D.; Wang, Y.; Wang, X. Effect of eleutheroside B1 on non-coding RNAs and protein profiles of influenza A virus-infected A549 cells. Int. J. Mol. Med. 2020, 45, 753–768. [Google Scholar] [CrossRef]
- Yan, W.; Zheng, C.; He, J.; Zhang, W.; Huang, X.A.; Li, X.; Wang, Y.; Wang, X. Eleutheroside B1 mediates its anti-influenza activity through POLR2A and N-glycosylation. Int. J. Mol. Med. 2018, 42, 2776–2792. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Andrographis paniculata (Burm. F) Wall ex Nees: Antiviral properties. Phytother. Res. 2021, 35, 5365–5373. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Wu, W.; Yang, J.; Yang, Z.; Liu, S. Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-κB and JAK-STAT signaling pathway. Microbes Infect. 2017, 19, 605–615. [Google Scholar] [CrossRef]
- Ko, H.C.; Wei, B.L.; Chiou, W.F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef]
- Yu, B.; Dai, C.Q.; Jiang, Z.Y.; Li, E.Q.; Chen, C.; Wu, X.L.; Chen, J.; Liu, Q.; Zhao, C.L.; He, J.X.; et al. Andrographolide as an Anti-H1N1 drug and the mechanism related to retinoic acid-inducible gene-I-like receptors signaling pathway. Chin. J. Integr. Med. 2014, 20, 540–545. [Google Scholar] [CrossRef]
- Sornpet, B.; Potha, T.; Tragoolpua, Y.; Pringproa, K. Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med. 2017, 10, 871–876. [Google Scholar] [CrossRef]
- Wintachai, P.; Kaur, P.; Lee, R.C.; Ramphan, S.; Kuadkitkan, A.; Wikan, N.; Ubol, S.; Roytrakul, S.; Chu, J.J.; Smith, D.R. Activity of andrographolide against chikungunya virus infection. Sci. Rep. 2015, 5, 14179. [Google Scholar] [CrossRef]
- Panraksa, P.; Ramphan, S.; Khongwichit, S.; Smith, D.R. Activity of andrographolide against dengue virus. Antivir. Res. 2017, 139, 69–78. [Google Scholar] [CrossRef]
- Ramalingam, S.; Karupannan, S.; Padmanaban, P.; Vijayan, S.; Sheriff, K.; Palani, G.; Krishnasamy, K.K. Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. Ayurveda 2018, 39, 87–91. [Google Scholar]
- Phumiamorn, S.; Sapsutthipas, S.; Pruksakorn, P.; Trisiriwanich, S. In Vitro Study on Antiviral Activity of Andrographis paniculata against COVID-19. 2020. Available online: https://www3.dmsc.moph.go.th/en/ (accessed on 21 December 2021).
- Sangiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Rajagopal, K.; Varakumar, P.; Baliwada, A.; Byran, G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): An in silico approach. Future J. Pharm. Sci. 2020, 6, 104. [Google Scholar] [CrossRef]
- Asea, A.; Kaur, P.; Wikman, K.G. Antiviral Activity and Synergy of Herba Andrographidis and Radix Eleutherococci Preparations against SARSCoV-2 Infected Vero E6 Human Primary Embryonic Kidney Epithelial Cells. J. Tradit. Complement. Med. 2021, 6, 148. [Google Scholar]
- Enmozhi, S.K.; Raja, K.; Sebastine, I.; Joseph, J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach. J. Biomol. Struct. Dyn. 2021, 39, 3092–3098. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Majumdar, M.; Singh, V.; Misra, T.K.; Roy, D.N. In silico studies on structural inhibition of SARS-CoV-2 main protease Mpro by major secondary metabolites of Andrographis paniculata and Cinchona officinalis. Biologia 2022, 77, 1373–1389. [Google Scholar] [CrossRef]
- Latha, D.; Hrishikesh, D.; Shiban, G.; Chandrashekar, C.; Bharath, B.R. In silico, in vitro screening of plant extracts for anti-SARS-CoV-2 activity and evaluation of their acute and sub-acute toxicity. Phytomed. Plus 2022, 2, 100233. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Janani, B.; Kannappan, P.; Renganathan, S.; Al-Ghamdi, S.; Alsaidan, M.; Abdelaziz, M.A.; Peer Mohideen, A.; Shahid, M.; Ramesh, T. In silico identification of potential inhibitors against main protease of SARS-CoV-2 6LU7 from Andrographis panniculata via molecular docking, binding energy calculations and molecular dynamics simulation studies. Saudi J. Biol. Sci. 2022, 29, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Wikman, G.; Efferth, T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine 2015, 22, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Klauck, S.M.; Efferth, T.; Panossian, A. Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy-induced cytotoxicity in neuroglia cells. Phytomedicine 2019, 58, 152743. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Klauck, S.M.; Efferth, T. Adaptogens in chemobrain (part IV): Adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells. Longhua Chin. Med. 2020, 3, 4. [Google Scholar]
- Narimanyan, M.; Jamalyan, K.; Balyan, A.; Barth, A.; Palm, S.; Wikman, G.; Panossian, A. Early intervention with Kan Jang® to treat upper-respiratory tract infections: A randomized, quadruple-blind study. J. Tradit. Complement. Med. 2021, 11, 552–562. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Efficacy of Andrographis paniculata in upper respiratory tract (URT) infectious diseases and the mechanism of action. In Evidence and Rational Based Research on Chinese Drugs; Wagner, H., Ulrich Merzenich, G., Eds.; Springer: Vienna, Austria, 2012; pp. 137–180. [Google Scholar]
- Coon, J.T.; Ernst, E. Andrographis paniculata in the treatment of upper respiratory tract infections: A systematic review of safety and efficacy. Planta Med. 2004, 70, 293–298. [Google Scholar]
- Cáceres, D.D.; Hancke, J.L.; Burgos, R.A.; Wikman, G.K. Prevention of common colds with Andrographis paniculata dried extract. A Pilot double blind trial. Phytomedicine 1997, 4, 101–104. [Google Scholar] [CrossRef]
- Gabrielian, E.S.; Shukarian, A.K.; Goukasova, G.I.; Chandanian, G.L.; Panossian, A.G.; Wikman, G.; Wagner, H. A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine 2002, 9, 589–597. [Google Scholar] [CrossRef]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang®: Decreases of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Kulichenko, L.L.; Kireyeva, L.; Malyshkina, E.N.; Wikman, G. A randomized, controlled study of Kan Jang® versus amantadine in the treatment of influenza in Volgograd. J. Herb. Pharmacother. 2003, 3, 77–93. [Google Scholar] [CrossRef]
- Melchior, J.; Palm, S.; Wikman, G. Controlled clinical study of standardized Andrographis paniculata extract in common cold—A pilot trial. Phytomedicine 1997, 3, 315–318. [Google Scholar] [CrossRef]
- Melchior, J.; Spasov, A.A.; Ostrovskij, O.V.; Bulanov, A.E.; Wikman, G. Double-blind, placebo-controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan Jang®) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine 2000, 7, 341–350. [Google Scholar] [CrossRef]
- Saxena, R.C.; Singh, R.; Kumar, P.; Yadav, S.C.; Negi, M.P.; Saxena, V.S.; Joshua, A.J.; Vijayabalaji, V.; Goudar, K.S.; Venkateshwarlu, K.; et al. A randomised double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185. [Google Scholar] [CrossRef]
- Spasov, A.A.; Ostrovskij, O.V.; Chernikov, M.V.; Wikman, G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother. Res. 2004, 18, 47–53. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf (accessed on 30 May 2022).
- Shang, Y.X.; Shen, C.; Stub, T.; Zhu, S.J.; Qiao, S.Y.; Li, Y.Q.; Wang, R.T.; Li, J.; Liu, J.P. Adverse Effects of Andrographolide Derivative Medications Compared to the Safe use of Herbal Preparations of Andrographis paniculata: Results of a Systematic Review and Meta-Analysis of Clinical Studies. Front. Pharmacol. 2022, 13, 773282. [Google Scholar] [CrossRef]
- Brekhman, I.I. On antitoxic action of Eleutherococcus. Mosc. Med. 1982, 37. [Google Scholar]
- Seo, E.J.; Klauck, S.M.; Efferth, T.; Panossian, A. Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy-induced toxicity-transcriptome-wide microarray analysis of neuroglia cells. Phytomedicine 2019, 56, 246–260. [Google Scholar] [CrossRef]
- HMPC—Committee on Herbal Medicinal Products. Assessment Report on Eleutherococcus senticosus (Rupr. et Maxim.) Maxim, Radix. EMA/HMPC/680615/2013. 25 March 2014, pp. 1–51. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-eleutherococcus-senticosus-rupr-et-maxim-maxim-radix_en.pdf (accessed on 30 May 2022).
- Lamb, Y.N. Nirmatrelvir Plus Ritonavir: First Approval. Drugs 2022, 82, 585–591. [Google Scholar] [CrossRef]
- Antiviral Drugs That Are Approved, Authorized, or under Evaluation for the Treatment of COVID-19. Last Updated: 29 April 2022. In COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. pp. 140–221. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_36.pdf (accessed on 29 April 2022).
- Molnupiravir_COVID19-MSD-Art 5(3)-Conditions for Use (Europa.eu). Available online: https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-conditions-use-conditions_en.pdf. (accessed on 7 August 2022).
- World Health Organization. Therapeutics and COVID-19. Therapeutic Management of Hospitalized Adults with COVID-19. Last Updated: 24 February 2022. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed on 7 August 2022).
- Lim, X.Y.; Chan, J.; Tan, T.Y.; Teh, B.P.; Mohd Abd Razak, M.R.; Mohamad, S.B.; Syed Mohamed, A.F. Andrographis paniculata (Burm. F.) Wall. Ex Nees, Andrographolide, and Andrographolide Analogues as SARS-CoV-2 Antivirals? A Rapid Review. Nat. Prod. Commun. 2021, 16, 1934578X211016610. [Google Scholar] [CrossRef]
- Izcovich, A.; Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Kum, E.; Qasim, A.; Khamis, A.M.; Rochwerg, B.; Agoritsas, T.; et al. Adverse effects of remdesivir, hydroxychloroquine and lopinavir/ritonavir when used for COVID-19: Systematic review and meta-analysis of randomised trials. BMJ Open 2022, 12, e048502. [Google Scholar] [CrossRef]
- Gupte, V.; Hegde, R.; Sawant, S.; Kalathingal, K.; Jadhav, S.; Malabade, R.; Gogtay, J. Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: A retrospective analysis of active surveillance database. BMC Infect. Dis. 2022, 22, 1. [Google Scholar] [CrossRef]









| Unit | Group A Kan Jang ADAPT n = 50 | Group B Placebo | Signif. of Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | n | Mean | SD. | n | Mean | SD. | p-Value | ||
| Age | years | 34 | 49.82 | 16.33 | 52 | 44.73 | 16.85 | 0.170 | |
| Gender | Male/Female | 34 | 12/22 (0.54) | 52 | 24/28 (0.86) | 0.156 | |||
| BMI | kg/m2 | 34 | 25.11 | 3.213 | 52 | 24.60 | 3.203 | 0.470 | |
| Start of symptoms | days | 34 | <3 | 52 | <3 | ||||
| Viral load, SARS-CoV2 | % | 34 | 100 | 52 | 100 | ||||
| Body temperature | °C | 34 | 37.6 | 0.39 | 52 | 37.6 | 0.44 | 0.903 | |
| Fatigue | 100% patients | A.U. | 34 | 1.77 | 0.47 | 52 | 1.79 | 0.53 | 0.403 |
| Headache | 85% patients | A.U. | 31 | 1.68 | 0.47 | 42 | 1.76 | 0.48 | 0.538 |
| Sore throat | 42% patients | A.U. | 18 | 1.500 | 0.514 | 18 | 1.500 | 0.618 | >0.999 |
| Cough | 38% patients | A.U. | 15 | 1.467 | 0.516 | 18 | 1.222 | 0.428 | 0.266 |
| Pain in muscles | 38% patients | A.U. | 11 | 1.636 | 0.674 | 22 | 1.773 | 0.429 | 0.314 |
| Runny nose | 12% patients | A.U. | 5 | 1.800 | 0.447 | 6 | 1.667 | 0.516 | >0.999 |
| Loss of smell | 8% patients | A.U. | 2 | 1.000 | 1.414 | 5 | 0.160 | 0.548 | 0.619 |
| Loss of taste | 0% of patients | A.U. | 0 | - | 0 | - | - | ||
| Physical activity | A.U. | 34 | 13.88 | 3.480 | 52 | 14.40 | 3.234 | 0.410 | |
| Physical activity (daily walk) | min | 34 | 8.824 | 10.94 | 52 | 14.13 | 15.33 | 0.120 | |
| Decreased attention (d2-test) | %E (errors) | 34 | 18.27 | 29.72 | 52 | 21.63 | 17.51 | 0.563 | |
| URTI | WI score | 34 | 13.21 | 4.241 | 52 | 12.06 | 4.425 | 0.235 | |
| QOL | WI score | 34 | 32.62 | 16.00 | 52 | 35.02 | 15.39 | 0.248 | |
| Blood serum IL-6 (normal level <7 pg/mL) | pg/mL | 34 | 19.50 * | 76.43 | 52 | 11.89 * | 20.46 | 0.738 | |
| D-dimer (normal range from 0.1 to 0.5 mg/L) | mg/L | 34 | 1.085 * | 2.033 | 52 | 5.94 * | 38.75 | 0.596 | |
| C-reactive protein (normal level <5 mg/L) | mg/L | 34 | 12.66 * | 12.81 | 52 | 18.65 * | 25.57 | 0.791 | |
| ALT (normal level <35 U/L) | U/L | 34 | 27.69 | 19.90 | 52 | 27.77 | 22.15 | 0.988 | |
| AST (normal level <32 U/L) | U/L | 34 | 27.82 | 24.08 | 52 | 27.62 | 22.41 | 0.956 | |
| Total WBC count, (normal range: 3.6–11.0 × 109 cells/L) | 109/L | 34 | 5.453 | 1.247 | 52 | 5.496 | 1.924 | 0.907 | |
| Erythrocytes, RBC (normal range: 3.8–5.8 × 1012 cells/L) | 1012/L | 34 | 4.764 | 0.479 | 52 | 13.60 * | 63.69 | 0.475 | |
| Hemoglobin. Hb (normal range 13.5–17.0 g/dl) | g/dl | 34 | 13.00 | 1.694 | 52 | 13.43 | 1.823 | 0.272 | |
| Hematocrit, HCT (normal range: 40–50, L/L) | L/L | 34 | 41.31 | 5.159 | 52 | 41.44 | 6.852 | 0.465 | |
| Platelet Count (normal range 150–380 × 103 cells/μL) | 103 μL | 34 | 194.6 | 45.90 | 52 | 204.3 | 49.57 | 0.336 | |
| Neutrophils count (normal range: 1.8–7.5 × 109 cells/L) | 109/L | 34 | 6.386 | 0.996 | 52 | 6.716 | 10.36 | 0.147 | |
| Lymphocyte count (normal range: 1.0–4.0 × 109 cells/L) | 109/L | 34 | 2.590 | 1.020 | 52 | 2.366 | 9.756 | 0.309 | |
| Monocyte count ((normal range: 0.1–1.0 × 109 cells/L) | 109/L | 34 | 1.035 * | 2.184 | 52 | 0.546 | 3.552 | 0.094 | |
| Eosinophil count ((normal range: 0.1–0.4 × 109 cells/L) | 109/L | 34 | 0.1268 | 0.111 | 52 | 0.0902 | 0.718 | 0.124 | |
| Basophil Count ((normal range: 0.01–0.1 × 109 cells/L) | 109/L | 34 | 0.0478 | 0.0261 | 52 | 0.0424 | 0.262 | 0.330 | |
| Herbal Extracts | Chemical Composition: Number of Compounds Identified in Extracts, n | Pharmacological Effect on Gene Expression in Target Cells: Number of Deregulated Genes in Host Cells, N |
|---|---|---|
| A—Andrographis | 39 | 207 |
| B—Eleutherococcus | 35 | 211 |
| C—Kan Jang combination | 74 | 250 |
| D—Andrographolide | 1 | 626 |
| Treatment | Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 Screening | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 14 | Day 21 | |
| Doctor’s visits | 1 Baseline | 2 | 3 | 4 | ||||
| Eligibility check/Information | * | |||||||
| Informed consent | * | |||||||
| Clinical examination | * | * | * | * | ||||
| Enrollment and allocation to intervention | * | |||||||
| Treatment (Kan Jang and placebo) | * | * | * | * | * | * | * | |
| Biomarker assessments | ||||||||
| Body temperature (fever) | * | * | * | * | * | * | * | * |
| COVID-19 PCR test | * | * | * | * | ||||
| Blood serum cytokine IL-6 (pg/mL) | * | * | * | * | ||||
| D-dimer (mg/L) | * | * | * | |||||
| C-reactive protein (mg/L) | * | * | * | |||||
| Blood cells count analysis | * | * | * | |||||
| ALT/AST | * | * | ||||||
| Clinician and observer reported outcomes assessments | ||||||||
| Cognitive performance (tests for attention and memory): d2 test | * | * | * | * | ||||
| Wisconsin URS Survey Score | * | * | * | * | ||||
| Drug intake accountability | * | |||||||
| Adverse events | * | * | * | |||||
| Patient-reported outcomes assessments | ||||||||
Mild COVID symptoms:
| * | * | * | * | * | * | * | * |
| Workout, min | * | * | * | * | ||||
| Physical activity (questionnaire) | * | * | * | * | ||||
| Paracetamol intake recording | * | * | * | * | * | * | * | |
| Rescue medication intake recording | * | * | * | * | * | * | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiani, L.; Pachkoria, E.; Mamageishvili, N.; Shengelia, R.; Hovhannisyan, A.; Panossian, A. Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15, 1013. https://doi.org/10.3390/ph15081013
Ratiani L, Pachkoria E, Mamageishvili N, Shengelia R, Hovhannisyan A, Panossian A. Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals. 2022; 15(8):1013. https://doi.org/10.3390/ph15081013
Chicago/Turabian StyleRatiani, Levan, Elene Pachkoria, Nato Mamageishvili, Ramaz Shengelia, Areg Hovhannisyan, and Alexander Panossian. 2022. "Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial" Pharmaceuticals 15, no. 8: 1013. https://doi.org/10.3390/ph15081013