Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H.Wigg from Călimani Mountains, Romania
Abstract
:1. Introduction
2. Results
2.1. Lichen Extracts
2.2. HPLC-DAD Determination of Usnic Acid Content
2.3. HPLC-DAD Determination of Polyphenols
2.4. Total Phenolic Content
2.5. Free-Radical Scavenging Activity Assay
2.6. Antibacterial Activity
2.7. Data Analysis
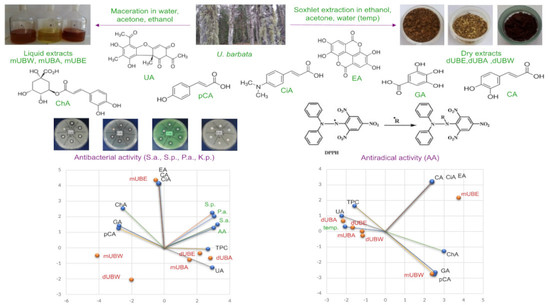
Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Lichen Extracts
4.3. HPLC-DAD Determination of Usnic Acid Content
4.3.1. Equipment and Chromatographic Conditions
4.3.2. Sample, Blank, Standard Solutions
4.3.3. Data Processing
4.4. HPLC-DAD Determination of Polyphenols
4.4.1. Equipment and Chromatographic Conditions
4.4.2. Sample, Blank, Standard Solutions
4.5. Total Phenolic Content
4.6. DPPH Free-Radical Scavenging Activity Assay
4.7. Antibacterial Activity
4.7.1. Microorganisms and Media
4.7.2. Inoculum Preparation
4.7.3. Lichen Samples Preparations
4.7.4. Disc Diffusion Method
4.7.5. Reading Plates
4.7.6. Interpretation of Disc Diffusion Method results
4.7.7. Activity Index
4.8. Data Analysis, Software
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, L.; Torres-Benítez, A.; Moreno-Palacios, M.; Simirgiotis, M.J.; Montoya-Serrano, S.A.; Sepulveda, B.; Stashenko, E.; García-Beltrán, O.; Areche, C. Phylogenetic Studies and Metabolite Analysis of Sticta Species from Colombia and Chile by Ultra-High Performance Liquid Chromatography-High Resolution-Q-Orbitrap-Mass Spectrometry. Metabolites 2022, 12, 560. [Google Scholar] [CrossRef] [PubMed]
- Stocker-Wörgötter, E.; Cordeiro, L.M.C.; Iacomini, M. Accumulation of potential pharmaceutically relevant lichen metabolites in lichens and cultured lichen symbionts. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 337–380. [Google Scholar]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.N.; Shrestha, M.; Pandey, D.P.; Bhattarai, T.; Bhattarai, H.D.; Paudel, B. Investigation of antioxidant, antimicrobial and toxicity activities of lichens from high altitude regions of Nepal. BMC Complement. Altern. Med. 2017, 17, 282. [Google Scholar] [CrossRef] [Green Version]
- Kello, M.; Kuruc, T.; Petrova, K.; Goga, M.; Michalova, Z.; Coma, M.; Rucova, D.; Mojzis, J. Pro-apoptotic potential of Pseudevernia furfuracea (L.) Zopf extract and isolated physodic acid in acute lymphoblastic leukemia model in vitro. Pharmaceutics 2021, 13, 2173. [Google Scholar] [CrossRef]
- Varol, M.; Tay, T.; Candan, M.; Türk, A.; Koparal, A.T. Evaluation of the sunscreen lichen substances usnic acid and atranorin. Biocell 2015, 39, 25–31. [Google Scholar]
- Varol, M. Lichens as a Promising Source of Unique and Functional Small Molecules for Human Health and Well-Being. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 60, pp. 425–458. [Google Scholar]
- Tas, I.; Yildirim, A.B.; Ozkan, E.; Ozyigitoglu, G.C.; Yavuz, M.Z.; Turker, A.U. Evaluation of pharmaceutical potential and phytochemical analysis of selected traditional lichen species. Farmacia 2021, 69, 1101–1106. [Google Scholar] [CrossRef]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A Comparative Study of Isolated Secondary Metabolites from Lichens and Their Antioxidative Properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Pillai Rajan, V.; Ramanathan, S.; Murugaiyah, V.; Samsudin, M.W.; Din, L. Antibacterial and Antioxidant Activity of Lichens Usnea rubrotincta, Ramalina dumeticola, Cladonia verticillata and Their Chemical Constituents. Malays. J. Anal. Sci. 2016, 20, 1–13. [Google Scholar] [CrossRef]
- Areche, C.; Parra, J.R.; Sepulveda, B.; Garc, O.; Simirgiotis, M.J. UHPLC-MS Metabolomic Fingerprinting, Antioxidant, and Enzyme Inhibition Activities of Himantormia lugubris from Antarctica. Metabolites 2022, 12, 560. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, L.; Fista, B.; Ismed, F.; Zaini, E. Membrane of Usnic Acid in Solid Dispersion and Effectiveness in Burn Healing. Adv. Health Sci. Res. 2021, 40, 323–329. [Google Scholar]
- Matvieieva, N.A.; Pasichnyk, L.A.; Zhytkevych, N.V.; Pabón, G.G.; Pidgorskyi, V.S. Antimicrobial Activity of Extracts from Ecuadorian Lichens. Mikrobiol. Z. 2015, 77, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.M.; Kim, Y.J.; Gang, H.S.; Han, J.; Ha, H.H.; Kim, H. Antimicrobial Activity of Divaricatic Acid Isolated from the Lichen Evernia mesomorpha against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 3068. [Google Scholar] [CrossRef] [Green Version]
- Fitriani, L.; Afifah; Ismed, F.; Bakhtiar, A. Hydrogel formulation of usnic acid and antibacterial activity test against Propionibacterium acne. Sci. Pharm. 2019, 87, 1. [Google Scholar] [CrossRef] [Green Version]
- Prateeksha Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.N.R.; Nayaka, S.; Joshi, Y.; Brahma Singh, N.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Oroian, M.; Mironeasa, S.; Schröder, V.; et al. Advances in the Characterization of Usnea barbata (L.) Weber ex F.H. Wigg from Călimani Mountains, Romania. Appl. Sci. 2022, 12, 4234. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef] [Green Version]
- Maulidiyah, M.; Darmawan, A.; Ahmad, E.; Musdalifah, A.; Wibowo, D.; Salim, L.O.A.; Arham, Z.; Mustapa, F.; Nurdin, I.F.A.; Nurdin, M. Antioxidant activity-guided isolation of usnic acid and diffractaic acid compounds from lichen genus Usnea sp. J. Appl. Pharm. Sci. 2021, 11, 075–083. [Google Scholar] [CrossRef]
- Bachtiar, E.; Hermawati, E.; Juliawaty, L.D.; Syah, Y.M. Antibacterial properties of usnic acid against vibriosis. Res. J. Chem. Environ. 2020, 24, 100–101. [Google Scholar]
- White, P.A.S.; Oliveira, R.C.M.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.S.; Gelain, D.P.; Moreira, J.C.F.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef] [PubMed]
- Kristmundsdóttir, T.; Jónsdóttir, E.; Ögmundsdóttir, H.M.; Ingólfsdóttir, K. Solubilization of poorly soluble lichen metabolites for biological testing on cell lines. Eur. J. Pharm. Sci. 2005, 24, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.P.; Wang, C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ. Toxicol. Pharmacol. 2020, 80, 103493. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.C.S.; Almeida, F.J.F.; Wanderley, M.S.O.; Ferraz, M.S.; Santos, N.P.S.; López, A.M.Q.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Usnic acid: From an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem. Rev. 2021, 20, 609–630. [Google Scholar] [CrossRef]
- Lira, M.C.B.; Ferraz, M.S.; da Silva, D.G.V.C.; Cortes, M.E.; Teixeira, K.I.; Caetano, N.P.; Sinisterra, R.D.; Ponchel, G.; Santos-Magalhães, N.S. Inclusion complex of usnic acid with β-cyclodextrin: Characterization and nanoencapsulation into liposomes. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 215–224. [Google Scholar] [CrossRef]
- Francolini, I.; Giansanti, L.; Piozzi, A.; Altieri, B.; Mauceri, A.; Mancini, G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf. B Biointerfaces 2019, 181, 632–638. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanoparticle Res. 2013, 15, 1766. [Google Scholar] [CrossRef]
- Baláž, M.; Goga, M.; Hegedüs, M.; Daneu, N.; Kováčová, M.; Tkáčiková, L.; Balážová, L.; Bačkor, M. Biomechanochemical Solid-State Synthesis of Silver Nanoparticles with Antibacterial Activity Using Lichens. ACS Sustain. Chem. Eng. 2020, 8, 13945–13955. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.; Husen, A.; Rehman, S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 23. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.H. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef]
- Mitrea, D.R.; Malkey, R.; Florian, T.L.; Filip, A.; Clichici, S.; Bidian, C.; Moldovan, R.; Hoteiuc, O.A.; Toader, A.M.; Baldea, I. Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J. Physiol. Pharmacol. 2020, 71, 74–81. [Google Scholar]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abozaid, O.A.R.; Moawed, F.S.M.; Ahmed, E.S.A.; Ibrahim, Z.A. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165904. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Song, F.; Jin, Y.C.; Zhang, W.M.; Zhang, Y.; Liu, E.J.; Zhou, D.; Bi, L.L.; Yang, Q.; Li, H.; et al. Comparative pharmacokinetics of gallic acid after oral administration of Gallic acid monohydrate in normal and isoproterenol-induced myocardial infarcted rats. Front. Pharmacol. 2018, 9, 328. [Google Scholar] [CrossRef] [Green Version]
- De Souza Tavares, W.; Pena, G.R.; Martin-Pastor, M.; de Sousa, F.F.O. Design and characterization of ellagic acid-loaded zein nanoparticles and their effect on the antioxidant and antibacterial activities. J. Mol. Liq. 2021, 341, 116915. [Google Scholar] [CrossRef]
- Cansaran, D.; Kahya, D.; Yurdakulol, E.; Atakol, O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Z. Fur Naturforsch.—Sect. C. J. Biosci. 2006, 61, 773–776. [Google Scholar] [CrossRef]
- Tosun, F.; Kizilay, Ç.A.; Şener, B.; Vural, M. The evaluation of plants from Turkey for in Vitro antimycobacterial activity. Pharm. Biol. 2005, 43, 58–63. [Google Scholar] [CrossRef]
- Bate, P.N.N.; Orock, A.E.; Nyongbela, K.D.; Babiaka, S.B.; Kukwah, A.; Ngemenya, M.N. In vitro activity against multi-drug resistant bacteria and cytotoxicity of lichens collected from Mount Cameroon. J. King Saud Univ.—Sci. 2020, 32, 614–619. [Google Scholar] [CrossRef]
- Zizovic, I.; Ivanovic, J.; Misic, D.; Stamenic, M.; Djordjevic, S.; Kukic-Markovic, J.; Petrovic, S.D. SFE as a superior technique for isolation of extracts with strong antibacterial activities from lichen Usnea barbata L. J. Supercrit. Fluids 2012, 72, 7–14. [Google Scholar] [CrossRef]
- Ivanovic, J.; Meyer, F.; Misic, D.; Asanin, J.; Jaeger, P.; Zizovic, I.; Eggers, R. Influence of different pre-treatment methods on isolation of extracts with strong antibacterial activity from lichen Usnea barbata using carbon dioxide as a solvent. J. Supercrit. Fluids 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Basiouni, S.; Fayed, M.A.A.; Tarabees, R.; El-Sayed, M.; Elkhatam, A.; Töllner, K.R.; Hessel, M.; Geisberger, T.; Huber, C.; Eisenreich, W.; et al. Characterization of sunflower oil extracts from the lichen Usnea barbata. Metabolites 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Ahmed, I.A.M.; Doǧu, S.; Uslu, N.; Gbemisola Jamiu, F.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M. The Effect of Heating Temperature on Total Phenolic Content, Antioxidant Activity, and Phenolic Compounds of Plum and Mahaleb Fruits. Int. J. Food Eng. 2019, 15, 11–12. [Google Scholar] [CrossRef]
- Humphries, R.M.; Abbott, A.N.; Hindler, J.A. Understanding and addressing CLSI breakpoint revisions: A primer for clinical laboratories. J. Clin. Microbiol. 2019, 57, e00203–e00219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiromi, P.S.A.I.; Hewawasam, R.P.; Jayalal, R.G.U.; Rathnayake, H.; Wijayaratne, W.M.D.G.B.; Wanniarachchi, D. Chemical Composition and Antimicrobial Activity of Two Sri Lankan Lichens, Parmotrema rampoddense, and Parmotrema tinctorum against Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureus. Evid.—Based Complement. Altern. Med. 2021, 9985325. [Google Scholar] [CrossRef]
- Farmacopeea Rom, 10th ed. 1993, pp. 419–421. Available online: https://ro.scribd.com/doc/215542717/Farmacopeea-Romana-X (accessed on 26 May 2022).
- Malik, J.; Mandal, S.C. Extraction of herbal biomolecules. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 21–46. [Google Scholar]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The Profile of Polyphenolic Compounds, Contents of Total Phenolics and Flavonoids, and Antioxidant and Antimicrobial Properties of Bee Products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef]
- Kosuru, R.Y.; Aashique, M.; Fathima, A.; Roy, A.; Bera, S. Revealing the dual role of gallic acid in modulating ampicillin sensitivity of Pseudomonas aeruginosa biofilms. Future Microbiol. 2018, 13, 297–312. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Schröder, V.; Costache, T.; Rambu, D.; Cucolea, I.E.; Gîrd, C.E.; Caraiane, A.; Gherghel, D.; et al. Antioxidant and cytotoxic activities of Usnea barbata (L.) F.H. Wigg. dry extracts in different solvents. Plants 2021, 10, 909. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Gîrd, C.E.; Rambu, D.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Ungureanu-Iuga, M.; Oroian, M.; Mironeasa, S.; et al. Antioxidant, Cytotoxic, and Rheological Properties of Canola Oil Extract of Usnea barbata (L.) Weber ex F. H. Wigg from Călimani Mountains, Romania. Plants 2022, 11, 854. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta—Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Maciag-Dorszyńska, M.; Wegrzyn, G.; Guzow-Krzemińska, B. Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol. Lett. 2014, 353, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Beema Shafreen, R.M.; Muthamil, S.; Karutha Pandian, S. Usnic acid, a lichen secondary metabolite inhibits Group A Streptococcus biofilms. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Gupta, V.K.; Kumar, P.; Kumar, R.; Joshi, R.; Pal, A.; Darokar, M.P. Usnic acid modifies MRSA drug resistance through down-regulation of proteins involved in peptidoglycan and fatty acid biosynthesis. FEBS Open Bio. 2019, 9, 2025–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef]
- Guan, S.; Zhu, K.; Dong, Y.; Li, H.; Yang, S.; Wang, S.; Shan, Y. Exploration of binding mechanism of a potential Streptococcus pneumoniae neuraminidase inhibitor from herbaceous plants by molecular simulation. Int. J. Mol. Sci. 2020, 21, 1003. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.; Abdel-Mawgoud, M.; Al-Sharary, T.; Almuhayawi, M.S.; Alruhaili, M.H.; Al Jaouni, S.K.; Warrad, M.; Mohamed, H.S.; Akhtar, N.; Abdelgawad, H. Pits of date palm: Bioactive composition, antibacterial activity and antimutagenicity potentials. Agronomy 2022, 12, 54. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Bren, U. Generation Times of E. coli Prolong with Increasing Tannin Concentration while the Lag Phase Extends Exponentially. Plants 2020, 9, 1680. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.P.; Bren, U. The effect of growth medium strength on minimum inhibitory concentrations of tannins and tannin extracts against E coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Z.H.; Wang, D.M.; Li, D.W.; Yang, L.N.; Wang, W. Chemical composition, antibacterial activity and related mechanism of valonia and shell from Quercus variabilis Blume (Fagaceae) against Salmonella paratyphi a and Staphylococcus aureus. BMC Complement. Altern. Med. 2019, 19, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V.; Falta, D.; Green, E. In-vitro antibacterial sensitivity of Usnea barbata lichen extracted with methanol and ethyl-acetate against selected Staphylococcus species from milk of cows with mastitis. Arch. Anim. Breed. 2014, 57, 25. [Google Scholar] [CrossRef] [Green Version]
- Mesta, A.R.; Rajeswari, N.; Kanivebagilu, V.S. Assessment of Antimicrobial Activity of Ethanolic Extraction of Usnea ghattensis and Usn Undulata. Int. J. Res. Ayurveda Pharm. 2020, 11, 75–77. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Evaluation of the Antibacterial Action of the Usnea barbata L. Extracts, on Streptococcus Species from the Oro-Dental Cavity. In Proceedings of the Romanian National Congress of Pharmacy, Bucharest, Romania, 26–29 September 2018. 17th ed.. [Google Scholar]
- Boitsova, T.A.; Brovko, O.S.; Ivakhnov, A.D.; Zhil’tsov, D.V. Optimizing Supercritical Fluid Extraction of Usnic Acid from the Lichen Species Usn Subfloridana. Russ. J. Phys. Chem. B 2020, 14, 1135–1141. [Google Scholar] [CrossRef]
- Stern, W.L.; Chambers, K.L. The Citation of Wood Specimens and Herbarium Vouchers in Anatomical. Int. Assoc. Plant Taxon. 2018, 9, 7–13. Available online: https://www.jstor.org/stable/1217349 (accessed on 20 May 2022). [CrossRef]
- Popovici, V.; Bucur, L.; Costache, T.; Gherghel, D.; Vochita, G.; Mihai, C.T.C.T.; Rotinberg, P.; Schroder, V.; Badea, F.C.F.C.; Badea, V.; et al. Studies on Preparation and UHPLC Analysis of the Usnea barbata (L.) F.H.Wigg Dry acetone extract. Rev. Chim. 2019, 70, 3775–3777. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [Green Version]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. and the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol Author Information. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Popovici, V.; Bucur, L.; Calcan, S.I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Schröder, V.; Gîrd, C.E.; Gherghel, D.; Vochita, G.; et al. Elemental Analysis and In Vitro Evaluation of Antibacterial and Antifungal Activities of Usnea barbata (L.) Weber ex F.H. Wigg from C ă limani Mountains, Romania. Plants 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Timm, M.; Saaby, L.; Moesby, L.; Hansen, E.W. Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology 2013, 65, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R.H. The use of XLSTAT in conducting principal component analysis (PCA) when evaluating the relationships between sensory and quality attributes in grilled foods. MethodsX 2020, 302, 125326. [Google Scholar] [CrossRef] [PubMed]











| Extraction Solvent | U. barbata Extract | Temperature of Extraction (°C) | Yield (%) | U. barbata Extract’s Color |
|---|---|---|---|---|
| Acetone | dUBA | 55–60 | 5.55 b | Yellow-brown |
| mUBA | 20–22 | n/a | Yellow | |
| Ethanol | dUBE | 75–80 | 11.15 a | Light brown |
| mUBE | 20–22 | n/a | Light brown | |
| Water | dUBW | 95–100 | 1.76 c | Dark brown-reddish |
| mUBW | 20–22 | n/a | Brown reddish |
| U. barbata Extract | UAC | ||
|---|---|---|---|
| mg/g Lichen Extract | mg/g Dried Lichen | ||
| Acetone | mUBA | 211.900 ± 0.002 b | 21.190 f |
| dUBA | 241.830 ± 0.172 a | 13.418 g | |
| Ethanol | mUBE | 0.257 ± 0.002 d | 0.025 i |
| dUBE | 108.742 ± 0.703 c | 12.125 h | |
| Water | mUBW | 0.045 ± 0.002 e | 0.004 j |
| dUBW | ND | n/a | |
| U. barbata Extracts | mUBE | mUBW | dUBE | dUBW |
|---|---|---|---|---|
| Polyphenols | Polyphenols Content mg/g Lichen Extract | |||
| Caffeic acid (CA) | 0.414 ± 0.005 | ND | ND | ND |
| p-coumaric acid (pCA) | 0.312 ± 0.001 b | 0.749 ± 0.049 a | ND | ND |
| Ellagic acid (EA) | 230.819 ± 0.264 c | ND | 0.605 ± 0.007 d | ND |
| Chlorogenic acid (ChA) | 0.512 ± 0.006 f | 0.627 ± 0.006 e | ND | ND |
| Gallic acid (GA) | 27.487 ± 0.459 h | 60.358 ± 0.363 g | 0.870 ± 0.008 k | ND |
| Cinnamic acid (CiA) | 17.948 ± 0.114 | ND | ND | ND |
| U. barbata Extract | TPC (mg PyE/g Extract) | DPPH-Free Radical Scavenging% | |
|---|---|---|---|
| Acetone | mUBA | 220.597 ± 24.527 d | 11.146 ± 0.577 k |
| dUBA | 862.843 ± 33.727 a | 15.471 ± 0.629 h | |
| Ethanol | mUBE | 276.603 ± 15.025 c | 12.162 ± 0.396 j |
| dUBE | 573.234 ± 42.308 b | 16.728 ± 0.284 g | |
| Water | mUBW | 176.129 ± 24.169 e | 6.429 ± 0.286 l |
| dUBW | 111.626 ± 11.132 f | 3.951 ± 0.297 m | |
| Bacteria | S. aureus | S. pneumoniae | P. aeruginosa | K. pneumoniae | |
|---|---|---|---|---|---|
| Inhibition Zone Diameter—IZD (mm) | |||||
| UA | 16.33 ± 0.82 | 17.33 ± 0.47 | 16.67 ± 0.47 | 0 | |
| Liquid extracts | |||||
| mUBA | 12.00 ± 0.82 b | 17.67 ± 0.47 | 17.33 ± 1.25 | 0 | |
| mUBE | 11.00 ± 0.82 d | 18.67 ± 0.47 | 20.33 ± 1.70 | 0 | |
| mUBW | 0 | 0 | 0 | 0 | |
| Dry extracts | |||||
| dUBA | 13.66 ± 0.47 a | 18.00 ± 1.63 | 17.00 ± 1.63 | 0 | |
| dUBE | 12.33 ± 1.25 c | 18.33 ± 0.47 | 20.00 ± 1.63 | 0 | |
| dUBW | 0 | 0 | 0 | 0 | |
| Standard antibacterial drugs inhibitory activity | |||||
| OFL 5 | 26.33 ± 1.70 | 19.00 ± 1.63 | 19.33 ± 1.70 | 30.00 ± 0.82 | |
| CTR 30 | 25.00 ± 2.45 | 32.33 ± 2.05 | 21.00 ± 2.16 | 32.33 ± 2.49 | |
| Standard antibacterial drugs breakpoints * | |||||
| Ofloxacin | |||||
| OFL 5 | S * | ≥18 * | ≥16 * | ≥16 * | ≥16 * |
| I * | 17–15 * | 15–13 * | 15–13 * | 15–13 * | |
| R * | ≤14 * | ≤12 * | ≤12 * | ≤12 * | |
| Ceftriaxone | |||||
| CTR 30 | S * | ≥21 * | ≥26 * | ≥18 * | ≥23 * |
| I * | 17–15 * | 22–20 * | |||
| R * | ≤20 * | ≤25 * | ≤14 * | ≤19 * | |
| Bacteria | AI Values (Adim) | AB | ||||
|---|---|---|---|---|---|---|
| mUBA | dUBA | mUBE | dUBE | UA | ||
| S. aureus | 0.455 | 0.519 | 0.417 | 0.468 | 0.620 | OFL5 |
| 0.480 | 0.546 | 0.440 | 0.490 | 0.693 | CTR30 | |
| S. pneumoniae | 0.930 a | 0.947 a | 0.982 a | 0.964 a | 0.912 a | OFL5 |
| 0.546 b | 0.556 b | 0.577 b | 0.566 b | 0.536 b | CTR30 | |
| P. aeruginosa | 0.896 | 0.879 | 1.051 | 1.034 | 0.862 | OFL5 |
| 0.825 | 0.809 | 0.968 | 0.952 | 0.793 | CTR30 | |
| U. barbata Extract | mUBE | dUBE | mUBA | dUBA | mUBW | dUBW | |
|---|---|---|---|---|---|---|---|
| Extraction conditions | |||||||
| Solvent | 96% ethanol | Acetone | Water | ||||
| Ratio (w/v) | 1:10 | ||||||
| Temperature (°C) | 20–22 | 75–80 | 20–22 | 55–60 | 20–22 | 95–100 | |
| Yield (%) | 11.150 | 5.550 | 1.760 | ||||
| Phenolic metabolites (mg/g extract) | |||||||
| TPC | 276.603 | 573.234 | 220.597 | 862.843 | 176.129 | 111.626 | |
| UA | mg/g extract | 0.257 | 108.74 | 211.190 | 241.830 | 0.045 | |
| % in dried lichen | 0.002 | 1.212 | 2.119 | 1.341 | 0.0004 | ||
| CA | 0.414 | ||||||
| pCA | 0.312 | 0.749 | |||||
| EA | 230.820 | 0.605 | |||||
| GA | 27.487 | 0.870 | 60.358 | ||||
| CiA | 17.948 | ||||||
| ChA | 0.513 | 0.627 | |||||
| Antibacterial activity—IZD (mm) | |||||||
| S.a. | 11.000 | 12.330 | 12.000 | 13.670 | |||
| S.p. | 18.670 | 18.330 | 17.670 | 18.000 | |||
| P.a. | 20.330 | 20.000 | 17.330 | 17.000 | |||
| DPPH free radical scavenging activity (%) | |||||||
| AA | 12.162 | 16.728 | 11.146 | 15.471 | 6.429 | 3.951 | |
| U. barbata Extract | Extraction Solvent | Conditions of Extraction | Yield % | UAC (mg/g in Extract) | % UA in Dried Lichen * | |||
|---|---|---|---|---|---|---|---|---|
| Pressure (Mpa) | Temperature (°C) | CO2 Pressure (m3/kg) | Pretreat- ment | |||||
| UBDEA a | Ethyl acetate | 75–80 | 6.27 | 376.73 | 2.362 | |||
| UB-SFE b | 99% CO2 | 30 | 60 | 0.38 | 594.80 | 2.226 | ||
| UB-SFE b | 99% CO2 | 30 | 40 | 0.60 | 364.90 | 2.190 | ||
| UBO c | Canola oil | 22 | 0.915 | 2.162 | ||||
| UBDA a | Acetone | 55–60 | 6.36 | 282.78 | 1.798 | |||
| UBDE a | 96% ethanol | 75–80 | 12.52 | 127.21 | 1.592 | |||
| UBDM a | Methanol | 65 | 11.29 | 137.60 | 1.553 | |||
| UB-SFE d | 99% CO2 | 50 | 40 | 992 | CM | 2.28 | 545 | 1.243 |
| RM | 1.67 | 585 | 0.977 | |||||
| UM + RGD | 1.50 | 645 | 0.968 | |||||
| 30 | 40 | 911 | UM | 1.27 | 617 | 0.806 | ||
| UM + RGD | 1.46 | 423 | 0.618 | |||||
| UM | 0.85 | 648 | 0.551 | |||||
| RM | 0.78 | 634.5 | 0.481 | |||||
| CM | 0.86 | 558.1 | 0.479 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, V.; Bucur, L.; Gîrd, C.E.; Popescu, A.; Matei, E.; Cozaru, G.C.; Schröder, V.; Ozon, E.A.; Fița, A.C.; Lupuliasa, D.; et al. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H.Wigg from Călimani Mountains, Romania. Pharmaceuticals 2022, 15, 829. https://doi.org/10.3390/ph15070829
Popovici V, Bucur L, Gîrd CE, Popescu A, Matei E, Cozaru GC, Schröder V, Ozon EA, Fița AC, Lupuliasa D, et al. Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H.Wigg from Călimani Mountains, Romania. Pharmaceuticals. 2022; 15(7):829. https://doi.org/10.3390/ph15070829
Chicago/Turabian StylePopovici, Violeta, Laura Bucur, Cerasela Elena Gîrd, Antoanela Popescu, Elena Matei, Georgeta Camelia Cozaru, Verginica Schröder, Emma Adriana Ozon, Ancuța Cătălina Fița, Dumitru Lupuliasa, and et al. 2022. "Phenolic Secondary Metabolites and Antiradical and Antibacterial Activities of Different Extracts of Usnea barbata (L.) Weber ex F.H.Wigg from Călimani Mountains, Romania" Pharmaceuticals 15, no. 7: 829. https://doi.org/10.3390/ph15070829