miR-182-5p Regulates Nogo-A Expression and Promotes Neurite Outgrowth of Hippocampal Neurons In Vitro
Abstract
:1. Introduction
2. Results
2.1. miR-182-5p Is Predicted to Regulate Mouse, Rat, and Human Nogo-A 3′UTRs
2.2. Nogo-A and miR-182-5p Expression in Neural Cell Lines
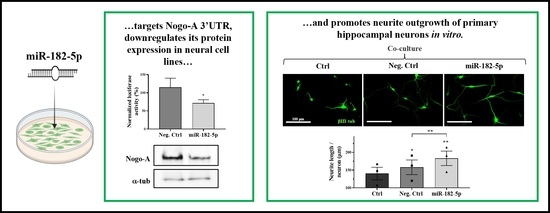
2.3. miR-182-5p Targets the Mouse Nogo-A 3′UTR and Downregulates Its Protein Expression
2.4. Nogo-A Downregulation by miR-182-5p Mimic Promotes Neurite Outgrowth of Rat Primary Hippocampal Neurons
3. Discussion
4. Materials and Methods
4.1. Bioinformatics and Data Mining
4.2. Cell Lines and Cultures
4.3. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.4. Vector Construction
4.5. Dual-Luciferase Reporter Assay
4.6. Immunoblot Assay
4.7. Neurite Outgrowth Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montani, L.; Gerrits, B.; Gehrig, P.; Kempf, A.; Dimou, L.; Wollscheid, B.; Schwab, M.E. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J. Biol. Chem. 2009, 284, 10793–10807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oertle, T.; Huber, C.; van der Putten, H.; Schwab, M.E. Genomic structure and functional characterisation of the promoters of human and mouse nogo/rtn4. J. Mol. Biol. 2003, 325, 299–323. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Oertle, T.; Schwab, M.E. Nogo and its paRTNers. Trends Cell Biol. 2003, 13, 187–194. [Google Scholar] [CrossRef]
- Josephson, A.; Widenfalk, J.; Widmer, H.W.; Olson, L.; Spenger, C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp. Neurol. 2001, 169, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.B.; Weinmann, O.; Brösamle, C.; Oertle, T.; Schwab, M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 2002, 22, 3553–3567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, D.; Coffin, R.S.; Prinjha, R.K.; Campbell, G.; Anderson, P.N. Nogo-A expression in the intact and injured nervous system. Mol. Cell. Neurosci. 2003, 24, 1083–1102. [Google Scholar] [CrossRef]
- Fournier, A.E.; GrandPre, T.; Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001, 409, 341–346. [Google Scholar] [CrossRef]
- Aloy, E.M.; Weinmann, O.; Pot, C.; Kasper, H.; Dodd, D.A.; Rülicke, T.; Rossi, F.; Schwab, M.E. Synaptic destabilization by neuronal Nogo-A. Brain Cell Biol. 2006, 35, 137–157. [Google Scholar] [CrossRef]
- Zemmar, A.; Chen, C.C.; Weinmann, O.; Kast, B.; Vajda, F.; Bozeman, J.; Isaad, N.; Schwab, M.E. Oligodendrocyte-and neuron-specific nogo-a restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb. Cortex. 2018, 28, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.J.; Scheel, T.K.; Luna, J.M.; Park, C.Y.; Fak, J.J.; Nishiuchi, E.; Rice, C.M.; Darnell, R.B. miRNA–target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat. Commun. 2015, 6, 8864. [Google Scholar] [CrossRef] [PubMed]
- Kocerha, J.; Kauppinen, S.; Wahlestedt, C. microRNAs in CNS disorders. Neuromol. Med. 2009, 11, 162–172. [Google Scholar] [CrossRef]
- Coolen, M.; Bally-Cuif, L. MicroRNAs in brain development and physiology. Curr. Opin. Neurobiol. 2009, 19, 461–470. [Google Scholar] [CrossRef]
- Wu, D.; Murashov, A.K. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front. Mol. Neurosci. 2013, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.C.; Zheng, J.Y.; Tang, L.J.; Huang, B.S.; Li, K.; Tao, Y.; Yu, W.; Zhu, R.L.; Li, S.; Li, L.X. MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell. Physiol. Biochem. 2015, 35, 246–258. [Google Scholar] [CrossRef]
- Gu, X.; Meng, S.; Liu, S.; Jia, C.; Fang, Y.; Li, S.; Fu, C.; Song, Q.; Lin, L.; Wang, X. miR-124 represses ROCK1 expression to promote neurite elongation through activation of the PI3K/Akt signal pathway. J. Mol. Neurosci. 2014, 52, 156–165. [Google Scholar] [CrossRef]
- Zhou, S.; Shen, D.; Wang, Y.; Gong, L.; Tang, X.; Yu, B.; Gu, X.; Ding, F. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef]
- Jia, S.; Qiao, X.; Ye, J.; Fang, X.; Xu, C.; Cao, Y.; Zheng, M. Nogo-C regulates cardiomyocyte apoptosis during mouse myocardial infarction. Cell Death Dis. 2016, 7, e2432. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhong, S.; Zhang, H.; Zhang, W.; Zhang, H.; Wu, X.; Chen, B. Prognostic value of MicroRNA-182 in cancers: A meta-analysis. Dis. Markers 2015, 2015, 482146. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Lei, R.; Hu, G. Roles of miR-182 in sensory organ development and cancer. Thorac. Cancer 2015, 6, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.M.; Lu, G.; Su, X.W.; Lyu, H.; Poon, W.S. MicroRNA-182 regulates neurite outgrowth involving the PTEN/AKT pathway. Front. Cell. Neurosci. 2017, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Bellon, A.; Iyer, A.; Bridi, S.; Lee, F.C.; Ovando-Vazquez, C.; Corradi, E.; Longhi, S.; Rocuzzo, M.; Strohbuecker, S.; Naik, S.; et al. miR-182 regulates Slit2-mediated axon guidance by modulating the local translation of a specific mRNA. Cell Rep. 2017, 18, 1171–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Qian, T.; Wang, Y.; Zhou, S.; Ding, G.; Ding, F.; Gu, X. miR-182 inhibits Schwann cell proliferation and migration by targeting FGF9 and NTM, respectively at an early stage following sciatic nerve injury. Nucleic Acids Res. 2012, 40, 10356–10365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennie, W.; Liu, C.; Carmack, C.S.; Wolenc, A.; Kanoria, S.; Lu, J.; Long, D.; Ding, Y. STarMir: A web server for prediction of microRNA binding sites. Nucleic Acids Res. 2014, 42, W114–W118. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.D.; Austin, J.W.; Fehlings, M.G.; Shoichet, M.S. A quantitative ELISA for bioactive anti-Nogo-A, a promising regenerative molecule for spinal cord injury repair. Methods 2009, 47, 104–108. [Google Scholar] [CrossRef]
- Hånell, A.; Clausen, F.; Björk, M.; Jansson, K.; Philipson, O.; Nilsson, L.N.; Hillered, L.; Weinreb, P.H.; Lee, D.; Mclntosh, T.K.; et al. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J. Neurotraum. 2010, 27, 1297–1309. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Shi, Y.; Cheng, S.; Yang, X.; Li, L.; Xiao, C. Nogo-A expresses on neural stem cell surface. Int. J. Neurosci. 2010, 120, 201–205. [Google Scholar] [CrossRef]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Meth. 2010, 186, 60–67. [Google Scholar] [CrossRef]
- Watanabe, E.; Hosokawa, H.; Kobayashi, H.; Murakami, F. Low Density, but not High Density, C6 Glioma Cells Support Dorsal Root Ganglion and Sympathetic Ganglion Neurite Growth. Eur. J. Neurosci. 1994, 6, 1354–1361. [Google Scholar] [CrossRef]
- Lingor, P.; Koch, J.C.; Tönges, L.; Bähr, M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 2012, 349, 289–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernet, V. Nogo-A in the visual system development and in ocular diseases. BBA-Mol. Basis Dis. 2017, 1863, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Zhang, N.; Xie, X.M.; Chen, Y.J.; Wang, R.; Shen, L.; Zhou, J.G.; Lü, H.Z. Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genom. 2017, 18, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Jia, Y.; Tang, W.; Cui, Q.; Liu, M.; Jiang, J. Roles of non-coding RNAs in central nervous system axon regeneration. Front. Neurosci. 2021, 15, 630633. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Diaz, M.; Esteban, F.J.; Reigada, D.; Muñoz-Galdeano, T.; Yunta, M.; Caballero-López, M.; Navarro-Ruiz, R.; del Águila, A.; Maza, R.M. MicroRNA dysregulation in spinal cord injury: Causes, consequences and therapeutics. Front. Cell. Neurosci. 2014, 8, 53. [Google Scholar] [CrossRef]
- Liu, N.K.; Wang, X.F.; Lu, Q.B.; Xu, X.M. Altered microRNA expression following traumatic spinal cord injury. Exp. Neurol. 2009, 219, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Yunta, M.; Nieto-Diaz, M.; Esteban, F.J.; Caballero-López, M.; Navarro-Ruíz, R.; Reigada, D.; Pita-Thomas, D.W.; del Águila, A.; Muñoz-Galdeano, T.; Maza, R.M. MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS ONE 2012, 7, e34534. [Google Scholar] [CrossRef]
- Li, F.; Zhou, M.W. MicroRNAs in contusion spinal cord injury: Pathophysiology and clinical utility. Acta Neurol. Belg. 2019, 119, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Fei, M.; Li, Z.; Cao, Y.; Jiang, C.; Lin, H.; Chen, Z. MicroRNA-182 improves spinal cord injury in mice by modulating apoptosis and the inflammatory response via IKKβ/NF-κB. Lab. Investig. 2021, 101, 1238–1253. [Google Scholar] [CrossRef]
- Wang, W.; Su, Y.; Tang, S.; Li, H.; Xie, W.; Chen, J.; Shen, L.; Pan, X.; Ning, B. Identification of noncoding RNA expression profiles and regulatory interaction networks following traumatic spinal cord injury by sequence analysis. Aging 2019, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Raafat, A.; Pak, E.; Clemens, S.; Murashov, A.K. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp. Neurol. 2012, 233, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higa, G.S.V.; de Sousa, E.; Walter, L.T.; Kinjo, E.R.; Resende, R.R.; Kihara, A.H. MicroRNAs in neuronal communication. Mol. Neurobiol. 2014, 49, 1309–1326. [Google Scholar] [CrossRef]
- Diao, H.J.; Low, W.C.; Lu, Q.R.; Chew, S.Y. Topographical effects on fiber-mediated microRNA delivery to control oligodendroglial precursor cells development. Biomaterials 2015, 70, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, A.; Barco, A. Role of Dicer and the miRNA system in neuronal plasticity and brain function. Neurobiol. Learn. Mem. 2016, 135, 3–12. [Google Scholar] [CrossRef]
- Theis, T.; Yoo, M.; Park, C.S.; Chen, J.; Kügler, S.; Gibbs, K.M.; Schachner, M. Lentiviral delivery of miR-133b improves functional recovery after spinal cord injury in mice. Mol. Neurobiol. 2017, 54, 4659–4671. [Google Scholar] [CrossRef]
- Yin, H.; Shen, L.; Xu, C.; Liu, J. Lentivirus-mediated overexpression of miR-29a promotes axonal regeneration and functional recovery in experimental spinal cord injury via PI3K/Akt/mTOR pathway. Neurochem. Res. 2018, 43, 2038–2046. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Pang, M.; Du, C.; Chen, Y.; Li, S.; Tian, Z.; Feng, F.; Wang, Y.; Chen, Z.; et al. MicroRNA-135a-5p Promotes the Functional Recovery of Spinal Cord Injury by Targeting SP1 and ROCK. Mol. Ther.-Nucl. Acids 2020, 22, 1063–1077. [Google Scholar] [CrossRef]
- Keller, A.; Gröger, L.; Tschernig, T.; Solomon, J.; Laham, O.; Schaum, N.; Viktoria, W.; Kern, F.; Schmartz, G.P.; Li, Y.; et al. miRNATissueAtlas2: An update to the human miRNA tissue atlas. Nucleic Acids Res. 2022, 50, D211–D221. [Google Scholar] [CrossRef]
- Strickland, E.R.; Hook, M.A.; Balaraman, S.; Huie, J.R.; Grau, J.W.; Miranda, R.C. MicroRNA dysregulation following spinal cord contusion: Implications for neural plasticity and repair. Neuroscience 2011, 186, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, Y. microRNA-182-5p alleviates spinal cord injury by inhibiting inflammation and apoptosis through modulating the TLR4/NF-κB pathway. Int. J. Clin. Exp. Patho. 2018, 11, 2948. [Google Scholar]
- Wang, J.W.; Yang, J.F.; Ma, Y.; Hua, Z.; Guo, Y.; Gu, X.L.; Zhang, Y.F. Nogo-A expression dynamically varies after spinal cord injury. Neural Regen. Res. 2015, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moya, S.M.; Ehses, J.; Kiebler, M.A. The alternative life of RNA—sequencing meets single molecule approaches. FEBS Lett. 2017, 591, 1455–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, C. Evolution and biological roles of alternative 3′ UTRs. Trends Cell Biol. 2016, 26, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Hir, H.; Gatfield, D.; Izaurralde, E.; Moore, M.J. The exon–exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001, 20, 4987–4997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, A.E.; Gomes, L.C.; Halder, R.; Jain, G.; Maass, F.; Tönges, L.; Tatenhorst, L.; Bähr, M.; Fischer, A.; Lingor, P. miR-182-5p and miR-183-5p act as GDNF mimics in dopaminergic midbrain neurons. Mol. Ther.-Nucl. Acids 2018, 11, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Li, S.; GrandPre, T.; Qiu, D.; Strittmatter, S.M. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 2003, 38, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Dimou, L.; Schnell, L.; Montani, L.; Duncan, C.; Simonen, M.; Schneider, R.; Liebscher, T.; Gullo, M.; Schwab, M.E. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J. Neurosci. 2006, 26, 5591–5603. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rønn, L.C.; Ralets, I.; Hartz, B.P.; Bech, M.; Berezin, A.; Berezin, V.; Møller, A.; Bock, E. A simple procedure for quantification of neurite outgrowth based on stereological principles. J. Neurosci. Meth. 2000, 100, 25–32. [Google Scholar] [CrossRef]




| Primers | Sequences (5′-3′) |
| 3′UTR-Nogo-A-wt | Forward: AACGAGCTCCATTCATCTTTAAAGGGGAC Reverse: ATATCTAGATTATGTCTATAT |
| 3′UTR-Nogo-A-mut | Forward: GTTAGAGAATTCATATAAGTAAATATAG Reverse: CTTATATGAATTCTCTAACAGTAAATC |
| pmiRGLO sequencing | CAAGAAGGGCGGCAAGATCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, A.; Nieto-Díaz, M.; Reigada, D.; Barreda-Manso, M.A.; Muñoz-Galdeano, T.; Maza, R.M. miR-182-5p Regulates Nogo-A Expression and Promotes Neurite Outgrowth of Hippocampal Neurons In Vitro. Pharmaceuticals 2022, 15, 529. https://doi.org/10.3390/ph15050529
Soto A, Nieto-Díaz M, Reigada D, Barreda-Manso MA, Muñoz-Galdeano T, Maza RM. miR-182-5p Regulates Nogo-A Expression and Promotes Neurite Outgrowth of Hippocampal Neurons In Vitro. Pharmaceuticals. 2022; 15(5):529. https://doi.org/10.3390/ph15050529
Chicago/Turabian StyleSoto, Altea, Manuel Nieto-Díaz, David Reigada, María Asunción Barreda-Manso, Teresa Muñoz-Galdeano, and Rodrigo M. Maza. 2022. "miR-182-5p Regulates Nogo-A Expression and Promotes Neurite Outgrowth of Hippocampal Neurons In Vitro" Pharmaceuticals 15, no. 5: 529. https://doi.org/10.3390/ph15050529