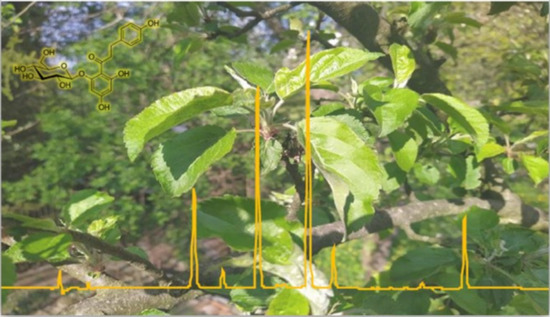
Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Chromatographic Separation Conditions
2.2. Optimization of Extraction Procedure
2.3. Validation of the Method
2.4. Determination of the Phenolic Profile in Apple Leaves, Bark, and Buds
2.5. Determination of Total Antioxidant Activity
2.6. Statistical Evaluation
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Raw Plant Material of Apple Trees
3.3. Preparation of the Standard Solutions for Method Optimization
3.4. Extraction of Phenolic Compounds
3.5. HPLC Equipment and Analysis
3.6. Validation of the Method
3.7. The Total Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Koninck, L. Ueber das Phloridzin (Phlorrhizin). In Annalen der Pharmacie, Band XV; Geiger, L.P., Liebig, J., Trommsdorff, J.B., Eds.; Universitäts-Buchhandlung von C. F. Winter: Heidelberg, Germany, 1835; pp. 75–77. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Martens, S.; Gang, D. Biosynthetic Pathway and Metabolic Engineering of Plant Dihydrochalcones. J. Agric. Food Chem. 2018, 66, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Lata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Viškelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Bílková, A.; Baďurová, K.; Svobodová, P.; Vávra, R.; Jakubec, P.; Chocholouš, P.; Švec, F.; Sklenářová, H. Content of major phenolic compounds in apples: Benefits of ultra-low oxygen conditions in long-term storage. J. Food Compos. Anal. 2020, 92, 103587. [Google Scholar] [CrossRef]
- Sowa, A.; Zgórka, G.; Szykuła, A.; Franiczek, R.; Żbikowska, B.; Gamian, A.; Sroka, Z. Analysis of Polyphenolic Compounds in Extracts from Leaves of Some Malus domestica Cultivars: Antiradical and Antimicrobial Analysis of These Extracts. Biomed. Res. Int. 2016, 2016, 6705431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Kumar, S.; Rana, A.; Sharma, V.; Katoch, P.; Padwad, Y.; Bhushan, S. Phenolic constituents from apple tree leaves and their in vitro biological activity. Ind. Crops Prod. 2016, 90, 118–125. [Google Scholar] [CrossRef]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. J. Agric. Food Chem. 2003, 51, 2896–2899. [Google Scholar] [CrossRef]
- Gosch, C.; Stich, K.; Halbwirth, H. Cloning and heterologous expression of glycosyltransferases from Malus x domestica and Pyrus communis, which convert phloretin to phloretin 20-O-glucoside (phloridzin). Plant Sci. 2010, 178, 299–306. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Pedreschi, R.; Yuan, J.; Kawas, J.R.; Chew, B.; Dowd, S.E.; Noratto, G. Apple consumption is associated with a distinctive microbiota, proteomics and metabolomics profile in the gut of Dawley Sprague rats fed a high-fat diet. PLoS ONE 2019, 14, e0212586. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirosaki, M.; Koyama, T.; Yazawa, K. Apple leaf extract as a potential candidate for suppressing postprandial elevation of the blood glucose level. J. Nutr. Sci. Vitaminol. 2012, 58, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Makarova, E.; Gornas, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute antihyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Ziaullah; Bhullar, K.S.; Warnakulasuriya, S.N.; Rupasinghe, H.P. Biocatalytic synthesis, structural elucidation, antioxidant capacity and tyrosinase inhibition activity of long chain fatty acid acylated derivatives of phloridzin and isoquercitrin. Bioorg. Med. Chem. 2013, 21, 684–692. [Google Scholar] [CrossRef]
- Jandera, P.; Škeříková, V.; Řehová, L.; Hájek, T.; Baldriánová, L.; Škopová, G.; Kellner, V.; Horna, A. RP-HPLC analysis of phenolic compounds and flavonoids in beverages and plant extracts using a CoulArray detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef]
- YMC. Available online: https://www.ymc.co.jp/en/columns/ymc_triart_series/ (accessed on 15 January 2022).
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Blasco, A.J.; Rogerio, M.C.; Gonzalez, M.C.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef] [PubMed]
- ICH. Available online: https://www.ich.org/page/quality-guidelines (accessed on 14 January 2022).




| Analyte | tR a (Min) | (r2) b | Calibration Range (mg/L) | Recovery (%) | Precision (RSD, %) | Repeatability (RSD, %) | LOD e (mg/L) | LOQ f (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 4.48 | 0.997 | 2–250 | 123.74 | 2.40 | 1.37; 0.72; 1.59 c | 0.078 | 0.260 |
| Rutin | 6.29 | 0.998 | 2–250 | 97.25 | 4.21 | 0.46; 0.91; 1.67 c | 0.145 | 0.434 |
| Quercitrin | 6.90 | 0.996 | 2–250 | 86.54 | 3.23 | 1.16; 0.90; 1.75 c | 0.146 | 0.482 |
| Phloridzin | 7.55 | 0.994 | 1000–8000 | 88.74 | 2.07 | 0.34; 0.51; 0.76 d | 0.080 | 0.263 |
| Phloretin | 10.52 | 0.998 | 2–250 | 90.44 | 4.56 | 0.47; 0.68; 1.62 c | 0.098 | 0.324 |
| Cultivar | All Phenolic Compounds | ||
|---|---|---|---|
| Leaves (mg/g ± SD) | Bark (mg/g ± SD) | Buds (mg/g ± SD) | |
| ‘Melrose’ | 98.93 ± 6.44 | 89.63 ± 1.45 | 105.82 ± 5.93 |
| ‘Melodie’ | 78.96 ± 11.81 | 81.18 ± 4.69 | 129.19 ± 11.10 |
| ‘James Grieve’ | 76.81 ± 3.65 | 99.86 ± 5.46 | 100.67 ± 7.21 |
| ‘Rubinola’ | 75.90 ± 3.04 | 89.26 ± 13.10 | 139.61 ± 4.52 |
| ‘Goldstar’ | 54.68 ± 10.38 | 83.62 ± 2.56 | - |
| ‘Meteor’ | 65.37 ± 7.92 | 70.81 ± 10.67 | - |
| ‘Průsvitné letní’ | 71.46 ± 2.80 | 78.75 ± 4.96 | 122.26 ± 1.19 |
| ‘Topaz’ | 106.81 ± 0.12 | 111.67 ± 3.48 | 133.01 ± 4.12 |
| ‘Red Bilt’ | 69.15 ± 4.05 | 101.35 ± 5.93 | 107.52 ± 4.77 |
| ‘Spartan’ | 103.07 ± 4.63 | 113.18 ± 2.60 | - |
| ‘Fragrance’ | 64.89 ± 1.27 | 100.64 ± 2.18 | 119.16 ± 6.76 |
| ‘Gloster’ | 104.72 ± 11.90 | 88.48 ± 2.32 | 101.05 ± 5.67 |
| ‘Bohemia Gold’ | 80.51 ± 6.26 | 84.69 ± 7.61 | 133.63 ± 11.47 |
| Cultivar | Phenolic Compound (mg/g ± SD) | ||||
|---|---|---|---|---|---|
| Phloridzin | Phloretin | Chlorogenic Acid | Rutin | Quercitrin | |
| ‘Melrose’ | 91.17 ± 2.15 | <LOQ | <LOQ | 3.07 ± 0.56 | 3.27 ± 3.73 |
| ‘Melodie’ | 66.83 ± 2.82 | <LOQ | 0.44 ± 2.14 | 2.14 ± 3.98 | 9.37 ± 2.87 |
| ‘James Grieve’ | 72.14 ± 0.27 | <LOD | 0.97 ± 0.51 | 0.70 ± 2.53 | 2.91 ± 0.34 |
| ‘Rubinola’ | 60.10 ± 0.01 | 0.37 ± 0.11 | 0.32 ± 0.83 | 3.83 ± 1.69 | 11.29 ± 0.41 |
| ‘Goldstar’ | 46.43 ± 0.06 | <LOQ | 0.37 ± 7.84 | 1.96 ± 1.86 | 5.80 ± 0.61 |
| ‘Meteor’ | 61.69 ± 1.69 | <LOQ | 0.48 ± 4.81 | 1.16 ± 1.35 | 1.89 ± 0.14 |
| ‘Průsvitné letní’ | 62.19 ± 0.26 | 0.35 ± 0.35 | 5.58 ± 1.39 | <LOQ | 2.94 ± 0.81 |
| ‘Topaz’ | 94.93 ± 0.08 | <LOQ | 1.18 ± 0.44 | 1.36 ± 1.75 | 9.18 ± 2.51 |
| ‘Red Bilt’ | 60.74 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | 1.33 ± 0.06 | 6.03 ± 0.05 |
| ‘Spartan’ | 98.51 ± 0.22 | 0.28 ± 0.40 | 1.08 ± 1.61 | 1.10 ± 0.10 | 2.11 ± 2.30 |
| ‘Fragrance’ | 53.80 ± 0.05 | <LOQ | <LOQ | 3.39 ± 0.60 | 7.29 ± 0.62 |
| ‘Gloster’ | 93.80 ± 0.80 | 0.54 ± 0.54 | 0.76 ± 1.30 | 3.60 ± 8.26 | 6.02 ± 0.97 |
| ‘Bohemia Gold’ | 71.70 ± 0.26 | <LOD | 0.80 ± 0.37 | 1.77 ± 4.64 | 6.15 ± 0.98 |
| Cultivar | Phenolic Compound (mg/g ± SD) | ||||
|---|---|---|---|---|---|
| Phloridzin | Phloretin | Chlorogenic Acid | Rutin | Quercitrin | |
| ‘Melrose’ | 76.59 ± 0.53 | nd | nd | 12.20 ± 0.64 | 0.84 ± 0.28 |
| ‘Melodie’ | 67.73 ± 0.09 | nd | nd | 11.41 ± 1.65 | 2.04 ± 2.95 |
| ‘James Grieve’ | 82.55 ± 0.59 | nd | nd | 16.03 ± 0.62 | 1.29 ± 4.26 |
| ‘Rubinola’ | 70.31 ± 0.70 | nd | nd | 16.54 ± 3.06 | 2.41 ± 9.35 |
| ‘Goldstar’ | 54.52 ± 0.40 | nd | <LOQ | 26.03 ± 1.29 | 2.85 ± 0.88 |
| ‘Meteor’ | 56.59 ± 1.70 | nd | nd | 13.49 ± 1.36 | 0.73 ± 7.60 |
| ‘Průsvitné letní’ | 57.48 ± 0.15 | nd | nd | 20.08 ± 0.29 | 1.19 ± 4.53 |
| ‘Topaz’ | 86.03 ± 0.41 | nd | nd | 22.58 ± 0.54 | 3.06 ± 2.53 |
| ‘Red Bilt’ | 85.62 ± 0.11 | nd | nd | 14.66 ± 0.60 | 1.07 ± 5.21 |
| ‘Spartan’ | 102.69 ± 0.55 | nd | nd | 12.29 ± 2.05 | - |
| ‘Fragrance’ | 82.50 ± 0.12 | nd | nd | 15.95 ± 70.71 | 2.19 ± 1.93 |
| ‘Gloster’ | 76.85 ± 0.17 | nd | nd | 10.68 ± 0.46 | 0.96 ± 1.69 |
| ‘Bohemia Gold’ | 65.08 ± 0.29 | nd | nd | 18.32 ± 2.38 | 1.28 ± 4.95 |
| Cultivar | Phenolic Compound (mg/g ± SD) | ||||
|---|---|---|---|---|---|
| Phloridzin | Phloretin | Chlorogenic Acid | Rutin | Quercitrin | |
| ‘Melrose’ | 94.41 ± 0.75 | nd | 3.48 ± 0.83 | 0.93 ± 2.39 | 7.00 ± 1.96 |
| ‘Melodie’ | 103.21 ± 1.34 | nd | 4.13 ± 1.33 | 3.22 ± 4.05 | 18.64 ± 4.39 |
| ‘James Grieve’ | 88.29 ± 1.27 | nd | 1.54 ± 1.21 | 2.41 ± 0.97 | 8.43 ± 3.76 |
| ‘Rubinola’ | 110.24 ± 0.67 | nd | 6.13 ± 0.73 | 2.80 ± 1.66 | 20.45 ± 1.46 |
| ‘Goldstar’ | - | - | - | - | - |
| ‘Meteor’ | - | - | - | - | - |
| ‘Průsvitné letní’ | 101.12 ± 0.55 | nd | 6.68 ± 0.51 | 1.93 ± 0.61 | 12.53 ± 0.23 |
| ‘Topaz’ | 111.68 ± 0.74 | nd | 1.93 ± 0.83 | 2.95 ± 2.00 | 16.45 ± 0.54 |
| ‘Red Bilt’ | 94.35 ± 1.20 | nd | 3.06 ± 1.32 | 1.80 ± 1.28 | 8.31 ± 0.96 |
| ‘Spartan’ | - | - | - | - | - |
| ‘Fragrance’ | 102.05 ± 1.43 | nd | 2.80 ± 1.43 | 1.12 ± 2.00 | 13.18 ± 1.90 |
| ‘Gloster’ | 88.51 ± 0.92 | nd | 2.69 ± 0.77 | 1.04 ± 2.16 | 8.82 ± 1.82 |
| ‘Bohemia Gold’ | 113.80 ± 2.85 | nd | 5.68 ± 3.17 | 3.07 ± 3.35 | 11.08 ± 2.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamcová, A.; Horna, A.; Šatínský, D. Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity. Pharmaceuticals 2022, 15, 244. https://doi.org/10.3390/ph15020244
Adamcová A, Horna A, Šatínský D. Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity. Pharmaceuticals. 2022; 15(2):244. https://doi.org/10.3390/ph15020244
Chicago/Turabian StyleAdamcová, Anežka, Aleš Horna, and Dalibor Šatínský. 2022. "Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity" Pharmaceuticals 15, no. 2: 244. https://doi.org/10.3390/ph15020244