Drug-Herb Interactions among Thai Herbs and Anticancer Drugs: A Scoping Review
Abstract
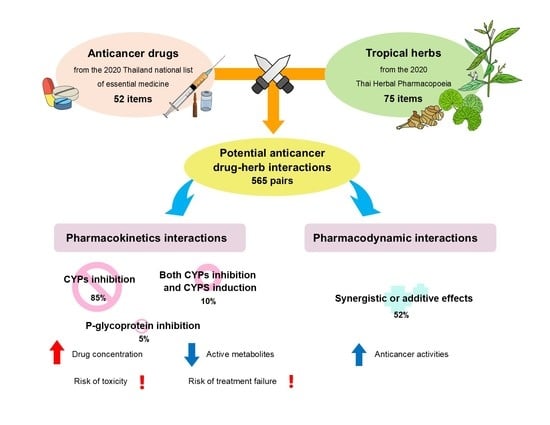
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Selection of Anticancer Drugs and Herbs
4.2. Criteria for the Literature Review
- (‘Scientific name of herbs’ OR ‘Common name of herbs’ OR ‘major components of herbs’);
- (‘In vitro’ OR ‘In vivo’ OR case reports OR clinical trials);
- (cytotoxicity OR antiproliferative activity OR anticancer);
- (Drug-herbs interaction OR Pharmacokinetic OR Pharmacodynamic);
- (‘anticancer drug name’)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 18 May 2021).
- World Health Organization. Thailand Fact Sheets. 2021. Available online: http://gco.iarc.fr/today/data/factsheets/populations/764-thailand-fact-sheets.pdf (accessed on 18 May 2021).
- World Health Organization. Cancer. 2021. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 18 May 2021).
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures. 2019–2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf (accessed on 18 May 2021).
- Balis, F.M. Pharmacokinetic drug interactions of commonly used anticancer drugs. Clin. Pharm. 1986, 11, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Alsanad, S.M.; Williamson, E.M.; Howard, R.L. Cancer patients at risk of herb/food supplement-drug interactions: A systematic review. Phytother. Res. 2014, 28, 1749–1755. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Samuels, N.; Goldstein, L.H.; Mutafoglu, K.; Omran, S.; Schiff, E.; Charalambous, H.; Dweikat, T.; Ghrayeb, I.; Bar-Sela, G.; et al. Potential risks associated with traditional herbal medicine use in cancer care: A study of Middle Eastern oncology health care professionals. Cancer 2016, 122, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Gougis, P.; Hilmi, M.; Geraud, A.; Mir, O.; Funck-Brentano, C. Potential cytochrome P450-mediated pharmacokinetic interactions between herbs, food, and dietary supplements and cancer treatments. Crit. Rev. Oncol. Hematol. 2021, 166, 103342. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Gubili, J.; Mao, J.J. Herb-drug interactions in cancer care. Oncology 2018, 32, 516–520. [Google Scholar]
- Sinuanchaeng, B.; Namvongprom, A.; Pakdevong, N.-O. Educative-supportive care needs, received and satisfaction among patients with early stage cancer. J. Nurs. Sci. Health 2018, 41, 24–33. [Google Scholar]
- Damery, S.; Gratus, C.; Grieve, R.; Warmington, S.; Jones, J.; Routledge, P.; Greenfield, S.; Dowswell, G.; Sherriff, J.; Wilson, S. The use of herbal medicines by people with cancer: A cross-sectional survey. Br. J. Cancer 2011, 104, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Thailand Development Research Institute. National List of Essential Medicines; Thailand Development Research Institute: Bangkok, Thailand, 2020. [Google Scholar]
- Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia 2020; Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health: Bangkok, Thailand, 2020.
- Manda, V.K.; Avula, B.; Khan, I.A.; Khan, S.I. Inhibitory effects of Aegle marmelos and its constituents on CYP3A4 and CYP1A2 in human liver microsomes. Planta Med. 2015, 81, PP10. [Google Scholar] [CrossRef]
- Abou-Diwan, C.; Ritchie, J. Drug interactions with garlic and ginger supplements. In Efficacy, Toxicity, Interactions with Western Drugs, and Effects on Clinical Laboratory Tests; Wiley: Hoboken, NJ, USA, 2011; pp. 333–350. [Google Scholar] [CrossRef]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging 2005, 22, 525–539. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Mehmood, T.; Zhang, Y.; Ma, T. Enhancing activity of anticancer drugs in multidrug resistant tumors by modulating P-glycoprotein through dietary nutraceuticals. Asian Pac. J. Cancer Prev. 2015, 16, 6831–6839. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, I.C.; Baek, H.S.; Moon, C.; Kim, S.H.; Yoo, J.C.; Shin, I.S.; Kim, J.C. Induction of cytochrome P450 3A1 expression by diallyl disulfide: Protective effects against cyclophosphamide-induced embryo-fetal developmental toxicity. Food Chem. Toxicol. 2014, 69, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Hatano, T. Effects of mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol. Pharm. Bull. 2010, 33, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, F.; Rose, C.; Ingvar, C.; Jernstrom, H. Natural remedies and hormone preparations—Potential risk for breast cancer patients. A study surveys the use of agents which possibly counteract with the treatment. Lakartidningen 2005, 102, 3226–3228, 3230–3231. [Google Scholar]
- Mooiman, K.D.; Maas-Bakker, R.F.; Hendrikx, J.J.M.A.; Bank, P.C.D.; Rosing, H.; Beijnen, J.H.; Schellens, J.H.M.; Meijerman, I. The effect of complementary and alternative medicines on CYP3A4-mediated metabolism of three different substrates: 7-benzyloxy-4-trifluoromethyl-coumarin, midazolam and docetaxel. J. Pharm. Pharmacol. 2014, 66, 865–874. [Google Scholar] [CrossRef]
- Williamson, E.M. Interactions between herbal and conventional medicines: The role of cytochrome P450 enzymes and P-glycoprotein. Pharmacologyonline 2006, 2, 200–205. [Google Scholar]
- Yang, L.J.; Fan, L.; Liu, Z.Q.; Mao, Y.M.; Guo, D.; Liu, L.H.; Tan, Z.R.; Peng, L.; Han, C.T.; Hu, D.L.; et al. Effects of allicin on CYP2C19 and CYP3A4 activity in healthy volunteers with different CYP2C19 genotypes. Eur. J. Clin. Pharmacol. 2009, 65, 601–608. [Google Scholar] [CrossRef]
- Zou, L.; Harkey, M.R.; Henderson, G.L. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002, 71, 1579–1589. [Google Scholar] [CrossRef]
- Bao, G.-Q.; Shen, B.-Y.; Pan, C.-P.; Zhang, Y.-J.; Shi, M.-M.; Peng, C.-H. Andrographolide causes apoptosis via inactivation of STAT3 and Akt and potentiates antitumor activity of gemcitabine in pancreatic cancer. Toxicol. Lett. 2013, 222, 23–35. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Jarukamjorn, K.; Kondo, S.; Nemoto, N. Synergistic increases of metabolism and oxidation–reduction genes on their expression after combined treatment with a CYP1A inducer and andrographolide. Chem. Biol. Interact. 2009, 182, 233–238. [Google Scholar] [CrossRef]
- Chen, S.; Hu, H.; Miao, S.; Zheng, J.; Xie, Z.; Zhao, H. Anti-tumor effect of cisplatin in human oral squamous cell carcinoma was enhanced by andrographolide via upregulation of phospho-p53 in vitro and in vivo. Tumor Biol. 2017, 39, 1010428317705330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Li, T.; Han, X.; Ren, J.; Chen, P.; Li, H.; Gong, S. The antitumor effect of arsenic trioxide on hepatocellular carcinoma is enhanced by andrographolide. Oncotarget 2017, 8, 90905–90915. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, Z.; Su, Z.; Sun, C.; Zhang, X.; Zhao, X.; Lai, X.; Su, Z.; Li, Y.; Zhan, J.Y. Enhanced anti-tumor activity and reduced toxicity by combination andrographolide and bleomycin in ascitic tumor-bearing mice. Eur. J. Pharmacol. 2016, 776, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hodroj, M.H.; Jardaly, A.; Abi Raad, S.; Zouein, A.; Rizk, S. Andrographolide potentiates the antitumor effect of topotecan in acute myeloid leukemia cells through an intrinsic apoptotic pathway. Cancer Manag. Res. 2018, 10, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S.; Hanapi, N.A.; Ab Halim, M.R.; Uchaipichat, V.; Mackenzie, P.I. Effects of Andrographis paniculata and Orthosiphon stamineus extracts on the glucuronidation of 4-methylumbelliferone in human UGT isoforms. Molecules 2010, 15, 3578–3592. [Google Scholar] [CrossRef] [Green Version]
- Jaruchotikamol, A.; Jarukamjorn, K.; Sirisangtrakul, W.; Sakuma, T.; Kawasaki, Y.; Nemoto, N. Strong synergistic induction of CYP1A1 expression by andrographolide plus typical CYP1A inducers in mouse hepatocytes. Toxicol. Appl. Pharmacol. 2007, 224, 156–162. [Google Scholar] [CrossRef]
- Jarukamjorn, K.; Don-in, K.; Makejaruskul, C.; Laha, T.; Daodee, S.; Pearaksa, P.; Sripanidkulchai, B.O. Impact of Andrographis paniculata crude extract on mouse hepatic cytochrome P450 enzymes. J. Ethnopharmacol. 2006, 105, 464–467. [Google Scholar] [CrossRef]
- Kang, X.; Zheng, Z.; Liu, Z.; Wang, H.; Zhao, Y.; Zhang, W.; Shi, M.; He, Y.; Cao, Y.; Xu, Q.; et al. Liposomal codelivery of doxorubicin and andrographolide inhibits breast cancer growth and metastasis. Mol. Pharm. 2018, 15, 1618–1626. [Google Scholar] [CrossRef]
- Lin, H.-H.; Shi, M.-D.; Tseng, H.-C.; Chen, J.-H. Andrographolide sensitizes the cytotoxicity of human colorectal carcinoma cells toward cisplatin via enhancing apoptosis pathways in vitro and in vivo. Toxicol. Sci. 2014, 139, 108–120. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.; He, P.; Wang, W.; Wu, X.; Wei, C. Andrographolide sensitizes Hep-2 human laryngeal cancer cells to carboplatin-induced apoptosis by increasing reactive oxygen species levels. Anticancer Drugs 2019, 30, 731–739. [Google Scholar] [CrossRef]
- Pan, Y.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Pook, P.C.; Er, H.M.; Ong, C.E. In vitro modulatory effects of Andrographis paniculata, Centella asiatica and Orthosiphon stamineus on cytochrome P450 2C19 (CYP2C19). J. Ethnopharmacol. 2011, 133, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Pekthong, D.; Blanchard, N.; Abadie, C.; Bonet, A.; Heyd, B.; Mantion, G.; Berthelot, A.; Richert, L.; Martin, H. Effects of Andrographis paniculata extract and andrographolide on hepatic cytochrome P450 mRNA expression and monooxygenase activities after in vivo administration to rats and in vitro in rat and human hepatocyte cultures. Chem. Biol. Interact. 2009, 179, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Pekthong, D.; Martin, H.; Abadie, C.; Bonet, A.; Heyd, B.; Mantion, G.; Richert, L. Differential inhibition of rat and human hepatic cytochrome P450 by Andrographis paniculata extract and andrographolide. J. Ethnopharmacol. 2008, 115, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Qin, B.; Liu, F.; Chen, Y.; Zhang, R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des. Dev. Ther. 2017, 11, 3333–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usia, T.; Iwata, H.; Hiratsuka, A.; Watabe, T.; Kadota, S.; Tezuka, Y. CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine 2006, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, B.; Gao, F.; Lan, M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm. Biol. 2016, 54, 2629–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunos, N.M.; Mutalip, S.S.M.; Jauri, M.H.; Yu, J.Q.; Huq, F. Anti-proliferative and pro-apoptotic effects from sequenced combinations of andrographolide and cisplatin on ovarian cancer cell lines. Anticancer Res. 2013, 33, 4365. [Google Scholar]
- Zhang, M.; Xue, E.; Shao, W. Andrographolide promotes vincristine-induced SK-NEP-1 tumor cell death via PI3K-AKT-p53 signaling pathway. Drug Des. Dev. Ther. 2016, 10, 3143–3152. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.P.; Li, W.; Xiao, X.F.; Zhang, L.L.; Liu, C.X. Phytochemical and pharmacological studies on Radix Angelica sinensis. Chin. J. Nat. Med. 2013, 11, 577–587. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, Y.Q.; Li, L.; Wang, Q.; Wang, N.S. Effect of Danggui and Honghua on cytochrome P450 1A2, 2C11, 2E1 and 3A1 mRNA expression in liver of rats. Am. J. Chin. Med. 2008, 36, 1071–1081. [Google Scholar] [CrossRef]
- Yu, C.; Chai, X.; Yu, L.; Chen, S.; Zeng, S. Identification of novel pregnane X receptor activators from traditional Chinese medicines. J. Ethnopharmacol. 2011, 136, 137–143. [Google Scholar] [CrossRef]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; Wink, M. Inhibition of cytochrome P450 (CYP3A4) activity by extracts from 57 plants used in Traditional Chinese Medicine (TCM). Pharmacogn. Mag. 2017, 13, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melillo de Magalhães, P.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ji, H.; Yang, B.; Ma, L.; Bei, Z.; Li, X.; Dang, H.; Yang, X.; Liu, C.; Wu, X.; et al. Impact of chrysosplenetin on the pharmacokinetics and anti-malarial efficacy of artemisinin against Plasmodium berghei as well as in vitro CYP450 enzymatic activities in rat liver microsome. Malar. J. 2015, 14, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pao, L.H.; Hu, O.Y.; Fan, H.Y.; Lin, C.C.; Liu, L.C.; Huang, P.W. Herb-drug interaction of 50 Chinese herbal medicines on CYP3A4 activity in vitro and in vivo. Am. J. Chin. Med. 2012, 40, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Sumsakul, W.; Mahavorasirikul, W.; Na-Bangchang, K. Inhibitory activities of Thai medicinal plants with promising activities against malaria and cholangiocarcinoma on human cytochrome P450. Phytother. Res. 2015, 29, 1926–1933. [Google Scholar] [CrossRef]
- Chen, J.-C.; Ko, J.-C.; Yen, T.-C.; Chen, T.-Y.; Lin, Y.-C.; Ma, P.-F.; Lin, Y.-W. Capsaicin enhances erlotinib-induced cytotoxicity via AKT inactivation and excision repair cross-complementary 1 (ERCC1) down-regulation in human lung cancer cells. Toxicol. Res. 2019, 8, 459–470. [Google Scholar] [CrossRef]
- Hong, Z.-F.; Zhao, W.-X.; Yin, Z.-Y.; Xie, C.-R.; Xu, Y.-P.; Chi, X.-Q.; Zhang, S.; Wang, X.-M. Capsaicin enhances the drug sensitivity of cholangiocarcinoma through the inhibition of chemotherapeutic-induced autophagy. PLoS ONE 2015, 10, e0121538. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, Y.; Yang, T.; Ma, X.; Cao, M.; Liu, L.; Yu, S.; Cao, A.; Liu, Y. Co-delivery of paclitaxel by a capsaicin prodrug micelle facilitating for combination therapy on breast cancer. Mol. Pharm. 2019, 16, 3430–3440. [Google Scholar] [CrossRef]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef]
- Sánchez, B.G.; Bort, A.; Mateos-Gómez, P.A.; Rodríguez-Henche, N.; Díaz-Laviada, I. Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Cancer Cell Int. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Deng, X.; Yu, C.; Zhao, G.; Zhou, J.; Zhang, G.; Li, M.; Jiang, D.; Quan, Z.; Zhang, Y. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J. Exp. Clin. Cancer Res. 2018, 37, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Huang, M.; Hu, H.; Yu, L.; Zeng, S. Pregnane X receptor-mediated transcriptional activation of UDP-glucuronosyltransferase 1A1 by natural constituents from foods and herbs. Food Chem. 2014, 164, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kulthong, K.; Tantisira, M.; Niwattisaiwong, N.; Apipalakul, K.; Chevapat, S.; Lawanprasert, S. Effects of the standard extract of Centella Asiatica (ECa233) on rat hepatic cytochrome P450. Thai J. Pharm. Sci. 2009, 33, 91–100. [Google Scholar]
- Pan, Y.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Pook, P.C.; Er, H.M.; Ong, C.E. In vitro modulatory effects on three major human cytochrome P450 enzymes by multiple active constituents and extracts of Centella asiatica. J. Ethnopharmacol. 2010, 130, 275–283. [Google Scholar] [CrossRef]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. In vitro assessment of CYP1A2 and 2C9 inhibition potential of Withania somnifera and Centella asiatica in human liver microsomes. Drug Metab. Pers. Ther. 2015, 30, 137–141. [Google Scholar] [CrossRef]
- Piyapolrungroi, N.; Sotanaphun, U.; Phattanawasin, P. Effect of leech lime juice on cytochrome P450 3A4 and P-glycoprotein activities. In Proceedings of the 3rd Asian Pacific Regional Meeting, Bangkok, Thailand, 10–12 May 2009; p. 122. [Google Scholar]
- Appiah-Opong, R.; Commandeur, J.N.; van Vugt-Lussenburg, B.; Vermeulen, N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef]
- Foster, B.C.; Vandenhoek, S.; Hana, J.; Krantis, A.; Akhtar, M.H.; Bryan, M.; Budzinski, J.W.; Ramputh, A.; Arnason, J.T. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine 2003, 10, 334–342. [Google Scholar] [CrossRef]
- Pouyfung, P.; Sarapusit, S.; Rongnoparut, P. Effects of Vernonia cinerea compounds on drug-metabolizing cytochrome P450s in human liver microsomes. Phytother. Res. 2017, 31, 1916–1925. [Google Scholar] [CrossRef]
- Han, Y.M.; Kim, I.S.; Rehman, S.U.; Choe, K.; Yoo, H.H. In vitro evaluation of the effects of Eurycoma longifolia extract on CYP-mediated drug metabolism. Evid. Based Complement. Altern. Med. 2015, 2015, 631329. [Google Scholar] [CrossRef] [Green Version]
- Muthiah, Y.D.; Ong, C.E.; Sulaiman, S.A.; Ismail, R. Inhibition of human cytochrome P450 2C8-catalyzed amodiaquine N-desethylation: Effect of five traditionally and commonly used herbs. Pharmacogn. Res. 2016, 8, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Langhammer, A.J.; Nilsen, O.G. In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother. Res. 2014, 28, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, A.J.; Nilsen, O.G. Fennel and raspberry leaf as possible inhibitors of acetaminophen oxidation. Phytother. Res. 2014, 28, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Jiang, J.; Qiu, F.; Liu, S.; Peng, P.; Gao, C.; Miao, P. Inhibitory effects of gypenosides on seven human cytochrome P450 enzymes in vitro. Food Chem. Toxicol. 2013, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.S.; Oyelola, F.T.; Ari, T.; Juho, H. In vitro inhibitory activities of the extract of Hibiscus sabdariffa L. (family Malvaceae) on selected cytochrome P450 isoforms. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumrongsakunchai, W.; Attakornvattana, V.; Somanabandhu, A.; Vannaprasaht, S.; Tassaneeyakul, W. Inhibitory effect and mechanism-based inhibition of Thai herbal plants on CYP3A4 and CYP2D6 activities. Thai J. Pharmacol. 2007, 29, 35–39. [Google Scholar]
- Suwannakul, S.; Kumpangngam, N.; Panprom, S.; Watcharathanakij, S.; Sethabouppha, B. Survey study of herbal medicine used in out-patients of Prasrimahabhodi psychiatric hospital, Thailand. In Proceedings of the The 7th Indochina Conference on Pharmaceutical Sciences: Advancing Pharmacy for ASEAN Community, Bangkok, Thailand, 14–16 December 2011; pp. 255–257. [Google Scholar]
- Noysang, C.; Mahringer, A.; Zeino, M.; Saeed, M.; Luanratana, O.; Fricker, G.; Bauer, R.; Efferth, T. Cytotoxicity and inhibition of P-glycoprotein by selected medicinal plants from Thailand. J. Ethnopharmacol. 2014, 155, 633–641. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.; Axson, C.; Vermeulen, N.P. Interactions between cytochromes P450, glutathione S-transferases and Ghanaian medicinal plants. Food Chem. Toxicol. 2008, 46, 3598–3603. [Google Scholar] [CrossRef]
- Konishi, T.; Satsu, H.; Hatsugai, Y.; Aizawa, K.; Inakuma, T.; Nagata, S.; Sakuda, S.H.; Nagasawa, H.; Shimizu, M. Inhibitory effect of a bitter melon extract on the P-glycoprotein activity in intestinal Caco-2 cells. Br. J. Pharmacol. 2004, 143, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Monera, T.G.; Wolfe, A.R.; Maponga, C.C.; Benet, L.Z.; Guglielmo, J. Moringa oleifera leaf extracts inhibit 6β-hydroxylation of testosterone by CYP3A4. J. Infect. Dev. Ctries. 2008, 2, 379–383. [Google Scholar] [CrossRef]
- Taesotikul, T.; Navinpipatana, V.; Tassaneeyakul, W. Selective inhibition of human cytochrome P450 1A2 by Moringa oleifera. Thai J. Pharmacol. 2010, 32, 256–258. [Google Scholar]
- Huang, L.; Bi, H.C.; Liu, Y.H.; Wang, Y.T.; Xue, X.P.; Huang, M. CAR-mediated up-regulation of CYP3A4 expression in LS174T cells by Chinese herbal compounds. Drug Metab. Pharm. 2011, 26, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limtrakul, P. Research and Development of Herbal Tea for Drug Resistance in Cervical Cancer; Department of Biochemistry Chiang Mai University: Bangkok, Thailand, 2003. [Google Scholar] [CrossRef]
- Liu, Y.H.; Mo, S.L.; Bi, H.C.; Hu, B.F.; Li, C.G.; Wang, Y.T.; Huang, L.; Huang, M.; Duan, W.; Liu, J.P.; et al. Regulation of human pregnane X receptor and its target gene cytochrome P450 3A4 by Chinese herbal compounds and a molecular docking study. Xenobiotica 2011, 41, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.H.; He, X.X.; Kong, L.T.; Liao, Y.H.; Pan, R.L.; Xiao, B.X.; Liu, X.M.; Chang, Q. Identification and characterization of potent CYP2D6 inhibitors in lotus leaves. J. Ethnopharmacol. 2014, 153, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hellum, B.H.; Liang, A.; Nilsen, O.G. The in vitro inhibition of human CYP1A2, CYP2D6 and CYP3A4 by tetrahydropalmatine, neferine and berberine. Phytother. Res. 2012, 26, 277–283. [Google Scholar] [CrossRef]
- Zhao, Y.; Hellum, B.H.; Liang, A.; Nilsen, O.G. Inhibitory mechanisms of human CYPs by three alkaloids isolated from traditional Chinese herbs. Phytother. Res. 2015, 29, 825–834. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Albassam, A.A.; Ahad, A.; Alsultan, A.; Al-Jenoobi, F.I. Inhibition of cytochrome P450 enzymes by thymoquinone in human liver microsomes. Saudi Pharm. J. 2018, 26, 673–677. [Google Scholar] [CrossRef]
- Dogar, M.Z.U.H.; Adi, H.; Akhtar, M.S.; Sheikh, M.A. Preliminary assessment of efficacy of Nigella sativa seeds in acute lymphoblastic leukemia in local children. Pharmacol. Online 2009, 2, 769–777. [Google Scholar]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the inhibitory effect of quercetin and thymoquinone on human cytochrome P450 enzyme activities. Pharm. Mag. 2017, 13, 895–899. [Google Scholar] [CrossRef]
- Khalife, R.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Thymoquinone from Nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro. Planta Med. 2016, 82, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aldebasy, Y.H.; Alsuhaibani, S.A.; Khan, M.A. Thymoquinone augments cyclophosphamide-mediated inhibition of cell proliferation in breast cancer cells. Asian Pac. J. Cancer Prev. 2019, 20, 1153–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Lv, X.; Liu, M.; Yang, Z.; Ji, M.; Guo, X.; Dong, W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem. Biophys. Res. Commun. 2012, 417, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.-G.; Zhang, L.-l.; Li, H.-Y.; Liao, Y.; Yu, H.-G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015, 60, 1067–1080. [Google Scholar] [CrossRef]
- Pan, Y.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Pook, P.C.; Er, H.M.; Ong, C.E. In vitro effects of active constituents and extracts of Orthosiphon stamineus on the activities of three major human cDNA-expressed cytochrome P450 enzymes. Chem. Biol. Interact. 2011, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sornsuvit, C.; Phosuya, C.; Jaroonwanichkul, D.; Piriyachananusorn, N. The use of herbal and dietary supplements and potential interactions with drugs in patients with chronic diseases. Thai Pharm. Health Sci. J. 2012, 7, 149–154. [Google Scholar]
- Anannarukan, N.; Niwattisaiwong, N.; Warisnoicharoen, W.; Winitthana, T.; Pramyothin, P.; Chaichantipayuth, C.; Lawanprasert, S. Inhibition of human cytochrome P450 in vitro by Phyllanthus amarus and Phyllanthus emblica aqueous extracts. Thai J. Pharm. Sci. 2012, 36, 135–143. [Google Scholar]
- Junyaprasert, V.B.; Soonthornchareonnon, N.; Thongpraditchote, S.; Murakami, T.; Takano, M. Inhibitory effect of Thai plant extracts on P-glycoprotein mediated efflux. Phytother. Res. 2006, 20, 79–81. [Google Scholar] [CrossRef]
- Pinmai, K.; Chunlaratthanabhorn, S.; Ngamkitidechakul, C.; Soonthornchareon, N.; Hahnvajanawong, C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: Doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J. Gastroenterol. 2008, 14, 1491–1497. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, K.; Bhadra, S.; Kar, A.; Bahadur, S.; Mitra, A.; Mukherjee, P.K. Cytochrome P450 inhibitory potential and RP-HPLC standardization of trikatu--a Rasayana from Indian Ayurveda. J. Ethnopharmacol. 2014, 153, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Han, H.K. Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J. Food Sci. 2010, 75, H93–H96. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.C.; Hathaway, L.B.; Lamb, J.G.; Pond, C.D.; Rai, P.P.; Matainaho, T.K.; Piskaut, P.; Barrows, L.R.; Franklin, M.R. Interactions of Papua New Guinea medicinal plant extracts with antiretroviral therapy. J. Ethnopharmacol. 2014, 155, 1433–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Dong, Z.; Dong, J.; Wei, L.; Shi, R.; Kang, S.; Zhang, D. Effects of mongolian medicine Terminalia chebula Retz. on 6 CYP450 enzymes in rats. Int. J. Clin. Exp. Pathol. 2020, 13, 3128–3138. [Google Scholar]
- Dumrongsakunchai, W.; Attakornvattana, V.; Somanabandhu, A.; Vannaprasaht, S.; Tassaneeyakul, W. Inhibitory effect of Thai herbal plants on CYP3A activity. Thai J. Pharmacol. 2006, 28, 88. [Google Scholar]
- Sompopcharoen, M.; Sresumatchai, V. Systematic Review: Marketing Communication of Thai Herbal Products to Enhance Potential in Becoming Global Products. In Proceedings of the The 1st International Conference on Innovative Communication and Sustainable Development in ASEAN, Bangkok, Thailand, 9–10 July 2015; pp. 243–253. [Google Scholar]
- Thai Herbal Product Champions. Available online: https://pharmacy.mahidol.ac.th/th/knowledge/article/404/ProductChampion%E0%B8%82%E0%B8%AD%E0%B8%87%E0%B8%AA%E0%B8%A1%E0%B8%B8%E0%B8%99%E0%B9%84%E0%B8%9E%E0%B8%A3%E0%B9%84%E0%B8%97%E0%B8%A2/ (accessed on 16 December 2020).
- Ye, L.-H.; Kong, L.-T.; Yan, M.-Z.; Cao, F.-R.; Wang, L.-S.; Liao, Y.-H.; Pan, R.-L.; Chang, Q. Lotus leaf alkaloid fraction can strongly inhibit CYP2D6 isoenzyme activity. J. Ethnopharmacol. 2016, 194, 913–917. [Google Scholar] [CrossRef]
- Bailey, D.G.; Dresser, G.; Arnold, J.M.O. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? CMAJ 2013, 185, 309–316. [Google Scholar] [CrossRef] [Green Version]
- AstraZeneca. Product Information: Nolvadex, Tamoxifen Citrate; AstraZeneca: North Ryde, Australia, 2003. [Google Scholar]
- BC Cancer Drug Manual. Tamoxifen. Available online: http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Tamoxifen_monograph.pdf (accessed on 18 May 2021).
- Mayne Pharma Group Limited. Product Information: SOLTAMOX Oral Solution, Tamoxifen Citrate Oral Solution; Mayne Pharma Group Limited: Salisbury South, Australia, 2018. [Google Scholar]
- Heel, R.C.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Tamoxifen: A review of its pharmacological properties and therapeutic use in the treatment of breast cancer. Drugs 1978, 16, 1–24. [Google Scholar] [CrossRef]
- Klein, D.J.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Tamoxifen pathway, pharmacokinetics. Pharmacogenet. Genom. 2013, 23, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Spitman, A.; Dezentje, V.; Swen, J.; Moes, D.; Bohringer, S.; Batman, E.; van Druten, E.; Smorenburg, C.; van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen pharmacogenetics and metabolism: Results from the prospective CYPTAM study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef]
- Junsaeng, D.; Anukunwithaya, T.; Songvut, P.; Sritularak, B.; Likhitwitayawuid, K.; Khemawoot, P. Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats. BMC Complement. Altern. Med. 2019, 19, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balis, F.M.; Holcenberg, J.S.; Bleyer, W.A. Clinical pharmacokinetics of commonly used anticancer drugs. Clin. Pharm. 1983, 8, 202–232. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.; McLeod, H.; Dolan, E.; Shukla, S.J.; Rabik, C.A.; Gong, L.; Hernandez-Boussard, T.; Lou, X.J.; Klein, T.E.; Altman, R.B. Platinum pathway. Pharm. Genom. 2009, 19, 563–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesarwani, K.; Gupta, R. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Hengjumrut, P.; Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Comparative pharmacokinetics between madecassoside and asiaticoside presented in a standardised extract of Centella asiatica, ECa 233 and their respective pure compound given separately in rats. Xenobiotica 2018, 48, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Songvut, P.; Chariyavilaskul, P.; Tantisira, M.H.; Khemawoot, P. Safety and pharmacokinetics of standardized extract of Centella asiatica (ECa 233) capsules in healthy Thai volunteers: A phase 1 clinical study. Planta Med. 2019, 85, 483–490. [Google Scholar] [CrossRef]
- Temeesak, N.; Kheokasem, N.; Phatcharawongsagorn, N.; Nontakulwiwat, P.; Boonmuang, P.; Santimaleeworagun, W.; Nulsopapon, P. The effects of herbs or dietary supplements on international normalized ratio in warfarin users: A retrospective study at Phramongkutklao hospital. Thai Pharm. Health Sci. J. 2015, 10, 139–146. [Google Scholar]
- Cox, M.C.; Low, J.; Lee, J.; Walshe, J.; Denduluri, N.; Berman, A.; Permenter, M.G.; Petros, W.P.; Price, D.K.; Figg, W.D.; et al. Influence of garlic (Allium sativum) on the pharmacokinetics of docetaxel. Clin. Cancer Res. 2006, 12, 4636–4640. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.H.; Yu, S.L.; Chen, H.Y.; Wang, C.C.; Chen, H.W.; Chen, J.J. The HLJ1-targeting drug screening identified Chinese herb andrographolide that can suppress tumour growth and invasion in non-small-cell lung cancer. Carcinogenesis 2013, 34, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Sheeja, K.; Guruvayoorappan, C.; Kuttan, G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int. Immunopharmacol. 2007, 7, 211–221. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuels, N.; Ben-Arye, E. Exploring herbal medicine use during palliative cancer care: The integrative physician as a facilitator of pharmacist–patient–oncologist communication. Pharmaceuticals 2020, 13, 455. [Google Scholar] [CrossRef] [PubMed]





| Thai Herbs | Potential Interactions | References |
|---|---|---|
| Acorus calamus |
| |
| Aegle marmelos |
| [14] |
| Albizia procera |
| |
| Allium ascalonicum |
| |
| Allium sativum |
| [15,16,17,18,19,20,21,22,23,24,25] |
| Andrographis paniculata |
| [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] |
| Anethum graveolens |
| [19] |
| Angelica dahurica |
| |
| Angelica sinensis |
| [46,47,48] |
| Arcangelisia flava |
| |
| Areca catechu |
| [49] |
| Artemisia annua |
| [49,50,51] |
| Atractylodes lancea |
| [52,53] |
| Aucklandia lappa |
| |
| Caesalpinia bonduc |
| |
| Capsicum annuum |
| [19,54,55,56,57,58,59] |
| Carum carvi |
| [19,60] |
| Cassia fistula |
| |
| Centella asiatica |
| [38,61,62,63] |
| Cissus quadrangularis |
| |
| Citrus hystrix |
| [64] |
| Clerodendrum indicum |
| |
| Clinacanthus nutans |
| |
| Cuminum cyminum |
| [19] |
| Curcuma longa |
| [19,65,66] |
| Curcuma spp. |
| |
| Cyanthillium cinereum (Vernonia cinerea) |
| [67] |
| Dracaena cochinchinensis |
| |
| Eurycoma longifolia |
| [68,69] |
| Ficus racemosa |
| |
| Foeniculum vulgare |
| [19,42,70,71] |
| Gynostemma pentaphyllum |
| [72] |
| Harrisonia perforata |
| |
| Hibiscus sabdariffa |
| [73] |
| Hyptis suaveolens |
| |
| Kaempferia parviflora |
| [74,75] |
| Lepidium sativum |
| |
| Ligusticum sinense |
| |
| Mesua ferrea |
| [76] |
| Mimusops elengi |
| |
| Momordica charantia |
| [17,77,78] |
| Moringa oleifera |
| [79,80] |
| Morus alba |
| [52,74,81,82,83] |
| Murdannia loriformis |
| |
| Nardostachys jatamansi |
| |
| Nelumbo nucifera |
| [84,85,86] |
| Neopicrorhiza scrophulariiflora |
| |
| Nigella sativa |
| [87,88,89,90,91,92,93,94,95] |
| Ocimum sanctum |
| |
| Orthosiphon aristatus (Orthosiphon stamineus) |
| [32,38,74,82,96,97] |
| Phyllanthus emblica |
| [98,99,100] |
| Pimpinella anisum |
| [19] |
| Piper betle |
| |
| Piper nigrum |
| [17,19,42,101,102] |
| Piper retrofractum |
| |
| Piper sarmentosum |
| |
| Piper wallichii |
| |
| Plantago ovata |
| |
| Pterocarpus santalinus |
| |
| Santalum album |
| [42] |
| Senna alata(Casssia alata) |
| [74,77,103] |
| Senna garrettiana(Cassia garrettiana) |
| |
| Senna tora(Cassia tora) |
| |
| Solanum trilobatum |
| [99] |
| Solori scandens(Derris scandens) |
| |
| Tarlmounia elliptica |
| |
| Terminalia bellirica |
| [100] |
| Terminalia chebula |
| [104] |
| Thunbergia laurifolia |
| [74,82,97,105] |
| Tiliacora triandra |
| |
| Tinospora crispa |
| [42] |
| Trachyspermum ammi |
| [19] |
| Zingiber montanum (Zingiber cassumunar) |
| [42] |
| Zingiber officinale |
| |
| Zingiber zerumbet(Zingiber aromaticum) |
| [42] |
| Thai Herbs | Effects of Thai Herbal Products | Potential Drug Interaction | Possible Effects on Anticancer Drugs | References |
|---|---|---|---|---|
| Aegle marmelos | CYP1A2 inhibition In vitro: Methanolic extract of Aegle marmelos inhibits CYP1A2 with IC50 = 0.8 μg/mL. | Dasatinib Imatinib | Increase concentrations | [14] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Methanolic extract of Aegle marmelos inhibits CYP3A4 in pooled human liver microsomes with IC50 = 5 μg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [14] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Allium sativum | CYP1A2 inhibition In vitro: Allicin inhibits CYP1A2 with IC50 = 44.22 µM. | Dasatinib Imatinib | Increase concentrations | [25] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Allicin, apigenin and myricetin inhibit CYP3A4 with IC50 = 43.73, 0.4, and 44.5 μM, respectively. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [20,25] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| CYP2C9 inhibition In vitro: Allicin, apigenin, and myricetin inhibit CYP2C9 with IC50 = 5.41, 6.4, and 32.1 μM, respectively. | Dasatinib Imatinib | Increase concentrations | [20,25] | |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP2C19 inhibition In vitro: Allicin inhibits CYP1A2 with IC50 = 3.52 µM. | Imatinib | Increase concentrations | [25] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Allicin inhibits CYP1A2 with IC50 = 47.10 µM. | Doxorubicin Imatinib | Increase concentrations | [25] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| Andrographis paniculata | CYP1A2 inhibition In vitro: Extract of Andrographis paniculata inhibits CYP1A2 with IC50 = 5.1 μg/mL. | Dasatinib Imatinib | Increase concentrations | [39,40] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP2C19 inhibition In vitro: Ethanolic extract of Andrographis paniculata inhibits CYP2C19 with IC50 = 91.7 μg/mL. | Imatinib | Increase concentrations | [38] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| UGT1A1 inhibition In vitro: Ethanolic extract of Andrographis paniculata inhibits UGT1A1 with IC50 = 5.00 µg/mL. | Etoposide Dasatinib | Increase concentrations | [32] | |
| UGT2B7 inhibition In vitro: Spray-dried 50% methanolic powder of Andrographis paniculata inhibits UGT2B7 with IC50 = 2.82 µg/mL. | Tamoxifen | Decrease levels of active metabolites | [32] | |
| Anethum graveolens | CYP3A4 inhibition In vitro: 100 µg/mL of Anethum graveolens extract inhibit CYP3A4 with percent inhibition more than 50%. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Angelica sinensis | CYP3A4 induction In vivo: Ethanolic crude extract, ligustilide, linoleic acid, ferulic acid, and beta-sitosterol from Angelica sinensis induces CYP3A4 activity in HepG2 cells with maximum induction at 118 ± 2.26% relative rifampin. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Decrease concentration | [48] |
| Cyclophosphamide Ifosfamide Tamoxifen | Increase levels of active metabolites | |||
| Areca catechu | CYP3A4 inhibition In vitro: 100 μg/mL of Areca catechu aqueous extracts inhibits CYP3A4 with percent inhibition 85% | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [49] |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Carum carvi | CYP2C9 inhibition In vitro: 100 μg/mL of Carum carvi extract inhibits CYP2C9 with percent inhibition more than 50%. | Dasatinib Imatinib | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: 100 μg/mL of Carum carvi extract inhibits CYP3A4 with percent inhibition more than 50%. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Centella asiatica | CYP2C19 inhibition In vitro: Dichloromethane extract of Centella asiatica inhibits CYP2C19 with IC50 = 30.2 μg/mL. | Imatinib | Increase concentrations | [38] |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2C9 inhibition In vitro: Ethanolic extract of Centella asiatica inhibits CYP2C9 with IC50 = 48.41 ± 4.64 μg/mL. | Dasatinib Imatinib | Increase concentrations | [63] | |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP1A2 inhibition In vitro: Ethanolic extract of Centella asiatica inhibits CYP1A2 with IC50 = 42.23 ± 3.65 μg/mL. | Dasatinib Imatinib | Increase concentrations | [63] | |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| Cuminum cyminum | CYP2C9 inhibition In vitro: 100 µg/mL of Cuminum cyminum extract inhibits CYP2C9 with percent inhibition more than 50%. | Dasatinib Imatinib | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: 100 µg/mL of Cuminum cyminum extract inhibits CYP3A4 with percent inhibition more than 75%. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Curcuma longa | CYP1A2 inhibition In vitro: Curcumin inhibits CYP1A2 with IC50 = 40 µM. | Dasatinib Imatinib | Increase concentrations | [65] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP2C9 inhibition In vitro: Curcumin inhibits CYP2C9 with IC50 = 14.8 µg/mL. Aqueous extract of Curcuma longa inhibits CYP2C9 with IC50 = 82.3 ± 6.05 µg/mL. | Dasatinib Imatinib | Increase concentrations | [19,66] | |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Extract of Curcuma longa inhibits CYP3A4 with IC50 = 17 µg/mL. Curcumin inhibits CYP3A4 with IC50 = 16.3 µM. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19,65] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Cyanthillium cinereum (Vernonia cinerea) | CYP2A6 inhibition In vitro: Flavonoid chrysoeriol inhibits CYP2A6 with Ki = 1.93 ± 0.05 µM, hirsutinolides inhibits CYP2A6 with IC50 = 12–23 μM. | Letrozole Tamoxifen | Increase concentrations | [67] |
| Ifosfamide | Decrease levels of active metabolites | |||
| CYP1A2 inhibition In vitro: Flavonoid chrysoeriol inhibits CYP1A2 with Ki = 3.39 ± 0.21 μM. | Dasatinib Imatinib | Increase concentrations | [67] | |
| Dacarbazine Flutamide | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Hirsutinolides inhibits CYP2D6 with IC50 = 15–41 μM. | Doxorubicin Imatinib | Increase concentrations | [67] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| Foeniculum vulgare | CYP2C9 inhibition In vitro: 100 µg/mL of Foeniculum vulgare extract inhibits CYP2C9 with percent inhibition more than 75%. | Dasatinib Imatinib | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Water extract of Foeniculum vulgare inhibits CYP2D6 with IC50 = 23 ± 2 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [70] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2E1 inhibition In vitro: Water extract of Foeniculum vulgare inhibits CYP2E1 with IC50 = 23 ± 4 µg/mL. | Dacarbazine Tamoxifen | Decrease levels of active metabolites | [71] | |
| CYP3A4 inhibition In vitro: 100 µg/mL of Foeniculum vulgare extract inhibits CYP3A4 with percent inhibition more than 75%, water extract of Foeniculum vulgare inhibits CYP3A4 with IC50 = 40 ± 4 µg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19,70] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Gynostemma pentaphyllum | CYP2D6 inhibition In vitro: Gypenosides inhibit CYP2D6 with IC50 = 1.61 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [72] |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2C8 inhibition In vitro: Gypenosides inhibit CYP2C8 with IC50 = 20.06 µg/mL. | Nilotinib Paclitaxel Tamoxifen | Increase concentrations | [72] | |
| Ifosfamide Imatinib | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Gypenosides inhibit CYP3A4 with IC50 = 34.76 µg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [72] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| CYP2C9 inhibition In vitro: Gypenosides inhibit CYP2C9 with IC50 = 54.52 µg/mL. | Dasatinib Imatinib Tamoxifen | Increase concentrations | [72] | |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| Kaempferia parviflora | CYP1A2 inhibition Patients who used extract from Kaempferia parviflora showed CYP1A2 inhibition. It also showed interaction with fluoxetine. | Dasatinib Imatinib | Increase concentrations | [75] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Ethanolic extract of Kaempferia parviflora inhibits CYP2D6 with IC50 = 77 ± 9.54 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [74] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Ethanolic extract of Kaempferia parviflora inhibits CYP3A4 with IC50 = 28 ± 19.5 µg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [74] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Moringa oleifera | CYP1A2 inhibition In vitro: Ethanolic extract inhibits CYP1A2 with IC50 = 13.8 ± 9.8 µg/mL. | Dasatinib Imatinib | Increase concentrations | [80] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| Nelumbo nucifera | CYP2C9 inhibition In vitro: Alkaloid fraction of Nelumbo nucifera inhibits CYP2C9 with IC50 = 52.58 µg/mL. | Dasatinib Imatinib | Increase concentrations | [84] |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP2C19 inhibition In vitro: Ethanolic extract of Nelumbo nucifera inhibits CYP2C19 with IC50 = 77.38 µg/mL. Alkaloid fraction of Nelumbo nucifera inhibits CYP2C19 with IC50 = 40.79 µg/mL. | Imatinib | Increase concentrations | [84] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Extract of Nelumbo nucifera inhibits CYP2D6 with IC50 = 12.05 µg/mL. Alkaloid fraction of Nelumbo nucifera inhibits CYP2D6 with IC50 = 0.96 µg/mL. In vivo: Alkaloid fraction of Nelumbo nucifera inhibits CYP2D6 in rat. | Doxorubicin Imatinib | Increase concentrations | [84,108] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Extract of Nelumbo nucifera inhibits CYP3A4 with IC50 = 15.7 ± 2.1 µg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [85] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Nigella sativa | CYP1A2 inhibition In vitro: Thymoquinone inhibits CYP1A2 with IC50 26.5 ± 2.9 µM | Dasatinib Imatinib | Increase concentrations | [88] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP2C9 inhibition In vitro: Thymoquinone inhibits CYP2C9 with IC50 0.5 ± 0.4 µM | Dasatinib Imatinib | Increase concentrations | [88] | |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Thymoquinone inhibits CYP3A4 with IC50 25.2 ± 3.1 µM | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [88] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| CYP2C19 inhibition In vitro: Thymoquinone inhibits CYP2C19 with IC50 3.6 ± 0.9 µM | Imatinib | Increase concentrations | [91] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| Orthosiphon aristatus (Orthosiphon stamineus) | CYP2C19 inhibition In vitro: Petroleum ether extract of Orthosiphon aristatus inhibits CYP2C19 with IC50 = 67.1 μg/mL. Sinensetin and eupatorin, active compounds of Orthosiphon aristatus, inhibit CYP2C19 with IC50 = 71.6 and 12.1 µg/mL, respectively. | Imatinib | Increase concentrations | [38] |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Ethanolic extract of Orthosiphon aristatus inhibits CYP2D6 with IC50 = 31.0 ± 19.5 µg/mL. Eupatorin, an active compound of Orthosiphon aristatus, inhibits CYP2D6 with IC50 = 3.8 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [74,96] | |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Dichloromethane and petroleum ether extracts of Orthosiphon aristatus inhibit CYP3A4 with IC50 = 96.5 and 46.3 µg/mL, respectively. Ethanolic extract of Orthosiphon aristatus inhibits CYP3A4 with IC50 = 40 ± 8.7 µg/mL. Rosmarinic acid and eupatorin, active compounds of Orthosiphon aristatus, inhibit CYP3A4 with IC50 = 86.9 and 5.0 µg/mL, respectively. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [74,96] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| UGT1A1 inhibition In vitro: Spray-dried 50% methanolic powder of Orthosiphon aristatus inhibits UGT1A1 with IC50 = 24.65 µg/mL. | Etoposide Dasatinib | Increase concentrations | [32] | |
| Pimpinella anisum | CYP3A4 inhibition In vitro: 100 µg/mL of Pimpinella anisum extract inhibits CYP3A4 with percent inhibition more than 50%. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Piper nigrum | CYP2C9 inhibition In vitro: Black pepper and white pepper extracts inhibit CYP2C9 with IC50 = 12.1 and 3.2 µg/mL, respectively. | Dasatinib Imatinib | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Black pepper and white pepper extracts inhibit CYP3A4 with IC50 = 4.1 and 1.0 µg/mL, respectively. Methanolic extract from Piper nigrum leaves and fruits inhibit CYP3A4 with IC50 = 25 and 29 µg/mL, respectively. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19,42] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Senna alata (Casssia alata) | CYP1A2 inhibition In vitro: Water extract powder of Senna alata inhibits CYP1A2 with IC50 = 28.3 ± 2.42 µg/mL. | Dasatinib Imatinib | Increase concentrations | [77] |
| Dacabarzine Flutamide | Decrease levels of active metabolites | |||
| CYP2D6 inhibition In vitro: Ethanolic extract of Senna alata inhibits CYP2D6 with IC50 = 33.0 ± 25.6 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [74,77] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Ethanolic extract of Senna alata inhibits CYP3A4 with IC50 = 24.3 ± 14.3 µg/mL. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [74] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Trachyspermum ammi | CYP3A4 inhibition In vitro: 100 µg/mL of Trachyspermum ammi extract inhibits CYP3A4 with percent inhibition more than 50%. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [19] |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Thunbergia laurifolia | CYP2D6 inhibition In vitro: Ethanolic extract of Thunbergia laurifolia inhibits CYP2D6 with IC50 = 45.0 ± 5.0 µg/mL. | Doxorubicin Imatinib | Increase concentrations | [74] |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites | |||
| Zingiber montanum (Zingiber cassumunar) | CYP2D6 inhibition In vitro: Extract of Zingiber montanum inhibits 25% of CYP2D6 when compare with Quinidine. | Doxorubicin Imatinib | Increase concentrations | [42] |
| Tamoxifen | Decrease levels of active metabolites | |||
| CYP3A4 inhibition In vitro: Extract of Zingiber montanum inhibits 50% of CYP3A4 when compare with Ketoclonazole. | Dasatinib Docetaxel Doxorubicin Etoposide Imatinib Letrozole Megestrol Nilotinib Paclitaxel Vinblastine Vincristine Vinorelbine | Increase concentrations | [42] | |
| Cyclophosphamide Ifosfamide Tamoxifen | Decrease levels of active metabolites |
| Anticancers in 2020 Thailand NLEM | Thai Herbs in 2020 THP |
|---|---|
Alkylating drugs
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiso, A.; Khemawoot, P.; Techapichetvanich, P.; Soopairin, S.; Phoemsap, K.; Damrongsakul, P.; Wongwiwatthananukit, S.; Vivithanaporn, P. Drug-Herb Interactions among Thai Herbs and Anticancer Drugs: A Scoping Review. Pharmaceuticals 2022, 15, 146. https://doi.org/10.3390/ph15020146
Jiso A, Khemawoot P, Techapichetvanich P, Soopairin S, Phoemsap K, Damrongsakul P, Wongwiwatthananukit S, Vivithanaporn P. Drug-Herb Interactions among Thai Herbs and Anticancer Drugs: A Scoping Review. Pharmaceuticals. 2022; 15(2):146. https://doi.org/10.3390/ph15020146
Chicago/Turabian StyleJiso, Apisada, Phisit Khemawoot, Pinnakarn Techapichetvanich, Sutinee Soopairin, Kittiphong Phoemsap, Panrawee Damrongsakul, Supakit Wongwiwatthananukit, and Pornpun Vivithanaporn. 2022. "Drug-Herb Interactions among Thai Herbs and Anticancer Drugs: A Scoping Review" Pharmaceuticals 15, no. 2: 146. https://doi.org/10.3390/ph15020146