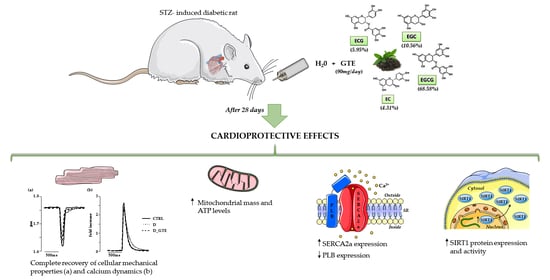
In Vivo Treatment with a Standardized Green Tea Extract Restores Cardiomyocyte Contractility in Diabetic Rats by Improving Mitochondrial Function through SIRT1 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Isolation of Adult Left Ventricular Cardiomyocytes
2.3. Cardiomyocyte Contractility and Calcium Transients
2.4. ATP Content Analysis in Isolated Cardiomyocytes
2.5. Citrate Synthase Activity Assay
2.6. SIRT1 Deacetylase Activity Assay
2.7. RNA Extraction, cDNA Synthesis and Relative Expression of Genes
2.8. Protein Extraction, SDS–Polyacrylamide Gel Electrophoresis (SDS–PAGE) and Western Blot (WB) Analysis
2.9. RNA Extraction and cDNA Synthesis
2.10. MiRNA Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Blood Glucose Levels and Body Weight
3.2. Cardiomyocyte Mechanics and Calcium Transients
3.3. ATP Content Analysis
3.4. Citrate Synthase Activity
3.5. RT-qPCR Analysis of SERCA2, PLB, CACNA1C, NCX1 and RYR2
3.6. Western Blot Analysis
3.7. SIRT1 Activity, Protein and mRNA Levels
3.8. MicroRNA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Bai, J.; Liu, C.; Zhu, P.; Li, Y. Novel Insights into Molecular Mechanism of Mitochondria in Diabetic Cardiomyopathy. Front. Physiol. 2021, 11, 609157. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; De la Fuente, S.; Palomeque, J.; Sheu, S.S. The role of mitochondria in metabolic disease: A special emphasis on heart dysfunction. J. Physiol. 2021, 599, 3477–3493. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, S.; Wang, J.; Xiao, M.; Guo, Y.; Tang, Y.; Zhang, J.; Gu, J. Epigenetic Regulation Associated with Sirtuin 1 in Complications of Diabetes Mellitus. Front. Endocrinol. 2021, 11, 598012. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Gorski, P.A.; Jang, S.P.; Jeong, D.; Lee, A.; Lee, P.; Oh, J.G.; Chepurko, V.; Yang, D.K.; Kwak, T.H.; Eom, S.H.; et al. Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca2+-ATPase in Heart Failure. Circ. Res. 2019, 124, e63–e80. [Google Scholar] [CrossRef]
- Ghosh, N.; Katare, R. Molecular mechanism of diabetic cardiomyopathy and modulation of microRNA function by synthetic oligonucleotides. Cardiovasc. Diabetol. 2018, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Al Hroob, A.M.; Abukhalil, M.H.; Hussein, O.E.; Mahmoud, A.M. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomed. Pharm. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Sala, R.; Frati, C.; Lagrasta, C.; Madeddu, D.; Falco, A.; Pollino, S.; Bresciani, L.; Miragoli, M.; et al. Parenchymal and Stromal Cells Contribute to Pro-Inflammatory Myocardial Environment at Early Stages of Diabetes: Protective Role of Resveratrol. Nutrients 2016, 8, 729. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Mena, P.; Dall’Asta, M.; Crozier, A.; Brighenti, F.; Stilli, D.; Del Rio, D. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Matta, M.J.; Sunderesan, N.R.; Gupta, M.P.; Periasamy, M.; Gupta, M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H833–H843. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-lasting partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y. Tea catechins and their applications as supplements and pharmaceutics. Pharmacol. Res. 2011, 64, 100–104. [Google Scholar] [CrossRef]
- Vilella, R.; Sgarbi, G.; Naponelli, V.; Savi, M.; Bocchi, L.; Liuzzi, F.; Righetti, R.; Quaini, F.; Frati, C.; Bettuzzi, S.; et al. Effects of Standardized Green Tea Extract and Its Main Component, EGCG, on Mitochondrial Function and Contractile Performance of Healthy Rat Cardiomyocytes. Nutrients 2020, 12, 2949. [Google Scholar] [CrossRef]
- Estrella, S.; Garcia-Diaz, D.F.; Codner, E.; Camacho-Guillén, P.; Pérez-Bravo, F. Expression of miR-22 and miR-150 in type 1 diabetes mellitus: Possible relationship with autoimmunity and clinical characteristics. Med. Clin. 2016, 147, 245–247. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef]
- Huang, Z.P.; Chen, J.; Seok, H.Y.; Zhang, Z.; Kataoka, M.; Hu, X.; Wang, D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013, 112, 1234–1243. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef]
- Rau, F.; Freyemuth, F.; Fugier, C.; Villemin, J.P.; Fischer, M.C.; Jost, B.; Dembele, D.; Gourdon, G.; Nicole, A.; Duboc, D.; et al. Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat. Struct. Mol. Biol. 2011, 18, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Lyon, A.R.; Volkmann, I.; Mills, A.M.; Bretthauer, J.; Pahuja, A.; Geers-Knörr, C.; Kraft, T.; Hajjar, R.J.; Macleod, K.T.; et al. SERCA2a gene therapy restores microRNA-1 expression in heart failure via an Akt/FoxO3A dependent pathway. Eur. Heart J. 2012, 33, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feng, Z.; Cheng, W.; Xiao, Y. MicroRNA-34a mediates atrial fibrillation through regulation of Ankyrin-B expression. Mol. Med. Rep. 2018, 17, 8457–8465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassani, J.W.; Bassani, R.A.; Bers, D.M. Relaxation in rabbit and rat cardiac cells: Species-dependent differences in cellular mechanisms. J. Physiol. 1994, 476, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Bocchi, L.; Savi, M.; Naponelli, V.; Vilella, R.; Sgarbi, G.; Baracca, A.; Solaini, G.; Bettuzzi, S.; Rizzi, F.; Stilli, D. Long-Term Oral Administration of Theaphenon-E Improves Cardiomyocyte Mechanics and Calcium Dynamics by Affecting Phospholamban Phosphorylation and ATP Production. Cell. Physiol. Biochem. 2018, 47, 1230–1243. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Li, Y.; Yu, S.; Zhao, Y. SIRT1/PGC-1α Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front. Mol. Neurosci. 2018, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Shyu, K.G.; Cheng, W.P.; Wang, B.W. Angiotensin II Downregulates MicroRNA-145 to Regulate Kruppel-like Factor 4 and Myocardin Expression in Human Coronary Arterial Smooth Muscle Cells under High Glucose Conditions. Mol. Med. 2015, 21, 616–625. [Google Scholar] [CrossRef]
- Wang, B.W.; Fang, W.J.; Shyu, K.G. MicroRNA-145 regulates disabled-2 and Wnt3a expression in cardiomyocytes under hyperglycaemia. Eur. J. Clin. Investig. 2018, 48, e12867. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wredenberg, A.; Wibom, R.; Wilhelmsson, H.; Graff, C.; Wiener, H.H.; Burden, S.J.; Oldfors, A.; Westerblad, H.; Larsson, N.G. Increased mitochondrial mass in mitochondrial myopathy mice. Proc. Natl. Acad. Sci. USA 2002, 99, 15066–15071. [Google Scholar] [CrossRef]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008, 77, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Guo, R.; Nair, S. Role of microRNA in diabetic cardiomyopathy: From mechanism to intervention. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2070–2077. [Google Scholar] [CrossRef]
- Vásquez-Reyes, S.; Velázquez-Villegas, L.A.; Vargas-Castillo, A.; Noriega, L.G.; Torres, N.; Tovar, A.R. Dietary bioactive compounds as modulators of mitochondrial function. J. Nutr. Biochem. 2021, 96, 108768. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, W.; Liu, J.; Gan, Y.; Liu, L.; Tian, J. Epigallocatechin-3 gallate prevents pressure overload-induced heart failure by up-regulating SERCA2a via histone acetylation modification in mice. PLoS ONE 2018, 13, e0205123. [Google Scholar] [CrossRef] [Green Version]
- Ca, Y.; Yu, S.S.; He, Y.; Bi, X.Y.; Gao, S.; Yan, T.D.; Zheng, G.D.; Chen, T.T.; Ye, J.T.; Liu, P.Q. EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol. 2021, 231, e13602. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Hamstra, S.I.; Kurgan, N.; Baranowski, R.W.; Qiu, L.; Watson, C.J.F.; Messner, H.N.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Fajardo, V.A. Low-dose lithium feeding increases the SERCA2a-to-phospholamban ratio, improving SERCA function in murine left ventricles. Exp. Physiol. 2020, 105, 666–675. [Google Scholar] [CrossRef]
- Koss, K.L.; Grupp, I.L.; Kranias, E.G. The relative phospholamban and SERCA2 ratio: A critical determinant of myocardial contractility. Basic Res. Cardiol. 1997, 92, 17–24. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxidative Med. Cell Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef] [Green Version]
- Nadtochiy, S.M.; Yao, H.; McBurney, M.W.; Gu, W.; Guarente, L.; Rahman, I.; Brookes, P.S. SIRT1-mediated acute cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1506–H1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, V.B.; Sundaresan, N.R.; Gupta, M.P. Regulation of Akt signaling by sirtuins: Its implication in cardiac hypertrophy and aging. Circ. Res. 2014, 114, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, L.; Ding, Y.; Yang, K.; Chen, Z.; Fan, X.; Jiang, S.; Guan, Y.; Liu, Z.; Xu, D.; et al. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future Med. Chem. 2018, 10, 795–809. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Perkins, G.; Murphy, A.N.; Naviaux, R.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: Effects of epicatechin rich cocoa. Clin. Trans. Sci. 2012, 5, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Feng, Z.; Liu, J.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Long, J.; Liu, J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: Ameliorating effects of (-)-epigallocatechin-3-gallate. J. Nutr. Biochem. 2012, 23, 716–724. [Google Scholar] [CrossRef]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; McAnulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef]
- Menzies, K.J.; Hood, D.A. The role of SirT1 in muscle mitochondrial turnover. Mitochondrion 2012, 12, 5–13. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Mastrocola, R.; Aragno, M.; Alloatti, G.; Collino, M.; Penna, C.; Pagliaro, P. Metaflammation: Tissue-Specific Alterations of the NLRP3 Inflammasome Platform in Metabolic Syndrome. Curr. Med. Chem. 2018, 25, 1294–1310. [Google Scholar] [CrossRef]







| Forward Primer Sequence | Reverse Primer Sequence | |
|---|---|---|
| SERCA2 | 5′ AACTACCTGGAGCCTGCAAT 3′ | 5′ TTCCCCAAGCTCAGTCATGC 3′ |
| PLN | 5′ CATGCCAACGCAGTTACAACCT 3′ | 5′ TCGTGACCCTTCACGACGAT 3′ |
| RYR2 | 5′ GGCGGAATTTCTTGCCAAC 3′ | 5′ CCTCGCACCTCATCCTGAGT 3′ |
| NCX1 | 5′ CGAAATGGATGGGAAAGTAGTCAAC 3′ | 5′ TCTTTGTCGGGATGCTTCTGC 3′ |
| CACNA1C | 5′ ATGGTTCTTGTCAGCATGTTGCGG 3′ | 5′ TGCAAATGTGGAACCGGTGAAGTG 3′ |
| SIRT1 | 5′ GTCTGTGCCTTCCAGTTGCT 3′ | 5′ CTGCTTGCTGTCCATACCTG 3′ |
| GAPDH | 5′ GTTCCAGAGACAGCCGCATC 3′ | 5′ CGTTCACACCGACCTTCACC 3′ |
| CTRL | D | D_GTE | |
|---|---|---|---|
| Day 0 | 103 ± 2 | 107 ± 6 | 105 ± 3 |
| Day 2 | 105 ± 4 | 356 ± 11 ** | 395 ± 28 ** |
| Day 14 | 101 ± 5 | 424 ± 25 ** | 448 ± 19 ** |
| Day 28 | 97 ± 5 | 472 ± 32 ** | 487 ± 3 ** |
| CTRL | D | D_GTE | |
|---|---|---|---|
| Day 0 | 359 ± 9 | 378 ± 5 | 384 ± 7 |
| Day 2 | 370 ± 9 | 355 ± 5 | 364 ± 8 |
| Day 14 | 382 ± 13 | 332 ± 6 ** | 335 ± 7 ** |
| Day 28 | 387 ± 14 | 331 ± 8 ** | 336 ± 9 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilella, R.; Izzo, S.; Naponelli, V.; Savi, M.; Bocchi, L.; Dallabona, C.; Gerra, M.C.; Stilli, D.; Bettuzzi, S. In Vivo Treatment with a Standardized Green Tea Extract Restores Cardiomyocyte Contractility in Diabetic Rats by Improving Mitochondrial Function through SIRT1 Activation. Pharmaceuticals 2022, 15, 1337. https://doi.org/10.3390/ph15111337
Vilella R, Izzo S, Naponelli V, Savi M, Bocchi L, Dallabona C, Gerra MC, Stilli D, Bettuzzi S. In Vivo Treatment with a Standardized Green Tea Extract Restores Cardiomyocyte Contractility in Diabetic Rats by Improving Mitochondrial Function through SIRT1 Activation. Pharmaceuticals. 2022; 15(11):1337. https://doi.org/10.3390/ph15111337
Chicago/Turabian StyleVilella, Rocchina, Simona Izzo, Valeria Naponelli, Monia Savi, Leonardo Bocchi, Cristina Dallabona, Maria Carla Gerra, Donatella Stilli, and Saverio Bettuzzi. 2022. "In Vivo Treatment with a Standardized Green Tea Extract Restores Cardiomyocyte Contractility in Diabetic Rats by Improving Mitochondrial Function through SIRT1 Activation" Pharmaceuticals 15, no. 11: 1337. https://doi.org/10.3390/ph15111337