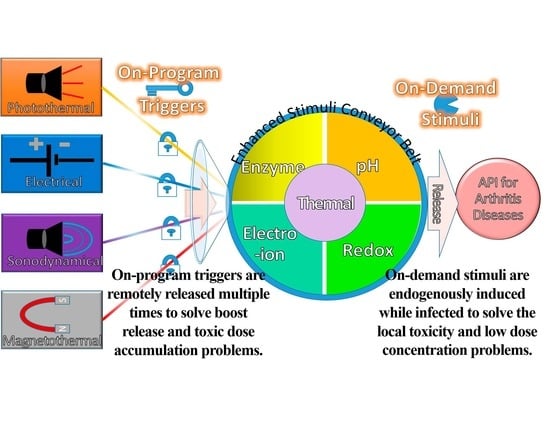
Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy
Abstract
:1. Introduction
2. Status Quo of Drug Carriers for Arthritis Therapy
2.1. Historical Development
2.2. Clinical Advancement
3. Pathological Alterations for Endogenous Stimuli
3.1. Enzymatic Stimulus
3.2. Redox Stimulus
3.3. Hyperthermia Stimulus
3.4. pH Stimulus
3.5. Electro-Ion Stimulus
4. Exogenous Triggers with Endogenous Stimuli
4.1. Photothermal Triggers
4.1.1. Photothermal Triggers Added Magnetic-Aid Targetor or Photoacoustic Detection
4.1.2. Photothermal Triggers Added Magnetic-Aid Targetor with pH or Redox Stimulus
4.2. Magnetothermal Triggers
Magnetothermal Triggers with Redox and pH Stimuli
4.3. Sonodynamical Triggers
Sonodynamical Triggers with Redox and Electro-Ion Stimuli
4.4. Electrical Triggers
4.4.1. Electrical Triggers with Metal-Organic Frameworks
4.4.2. Electrical Triggers with Conductive Polymers
4.4.3. Electrical Trigger with Enzymatic Stimulus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wu, C.; Cheng, J.; Li, W.; Yang, L.; Dong, H.; Zhang, X. Programmable Polymeric Microneedles for Combined Chemotherapy and Antioxidative Treatment of Rheumatoid Arthritis. ACS Appl. Mater. Interfaces 2021, 13, 55559–55568. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, H.K.; Susin, S.A. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist. Updates 2007, 10, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Michallek, F.; Ulas, S.T.; Poddubnyy, D.; Proft, F.; Schneider, U.; Hermann, K.-G.A.; Dewey, M.; Diekhoff, T. Fractal analysis of perfusion imaging in synovitis: A novel imaging biomarker for grading inflammatory activity based on assessing angiogenesis. RMD Open 2022, 8, e002078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, L.; Zhou, H.; Li, M.; Wang, J.; Guo, L. Application of Dynamic Contrast-Enhanced MRI in the Diagnosis of Rheumatoid Arthritis. Contrast Media Mol. Imaging 2022, 2022, 3055465. [Google Scholar] [CrossRef] [PubMed]
- Medrado, L.N.; Mendonça, M.L.M.; Budib, M.B.; Oliveira-Junior, S.A.; Martinez, P.F. Effectiveness of aquatic exercise in the treatment of inflammatory arthritis: Systematic review. Rheumatol. Int. 2022, 42, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, R.; Buchanan, W.W. Osteoarthritis: Symptoms, signs and source of pain. Inflammopharmacology 2004, 12, 3–31. [Google Scholar] [CrossRef]
- Li, Z.A.; Sant, S.; Cho, S.K.; Goodman, S.B.; Bunnell, B.A.; Tuan, R.S.; Gold, M.S.; Lin, H. Synovial joint-on-a-chip for modeling arthritis: Progress, pitfalls, and potential. Trends Biotechnol. 2022. [Google Scholar] [CrossRef]
- de la Torre-Aboki, J.; Uson, J.; Pitsillidou, I.; Vardanyan, V.; Nikiphorou, E.; Rodriguez-Garcia, S.C.; Castellanos-Moreira, R.; Pandit, H.; O’Neill, T.W.; Doherty, M.; et al. Intra-articular therapies: Patient preferences and professional practices in European countries. Rheumatol. Int. 2022, 42, 869–878. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, W.; You, T.; Zhang, X.; Zhang, W.; Li, C. Effectiveness of arthroscopic distal clavicle resection for symptomatic acromioclavicular joint arthritis. Chin. J. Reparative Reconstr. Surg. 2022, 36, 698–702. [Google Scholar] [CrossRef]
- Wolford, L.M.; Kesterke, M.J. Does Combined Temporomandibular Joint Reconstruction With Patient-Fitted Total Joint Prosthesis and Orthognathic Surgery Provide Stable Skeletal and Occlusal Outcomes in Juvenile Idiopathic Arthritis Patients? J. Oral Maxillofac. Surg. 2022, 80, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. Structural Strength Analyses for Low Brass Filler Biomaterial with Anti-Trauma Effects in Articular Cartilage Scaffold Design. Materials 2022, 15, 4446. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Jahan, R.; Paul, A.; Mahboob, T.; Bondhon, T.A.; Jannat, K.; Hasan, A.; Nissapatorn, V.; Wilairatana, P.; de Lourdes Pereira, M.; et al. The Role of Medicinal and Aromatic Plants against Obesity and Arthritis: A Review. Nutrients 2022, 14, 985. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, J.; Guo, Y.; Gu, L.; Wu, P.; Tong, M.; Liu, C.; Sun, J. B7-H3 blockade decreases macrophage inflammatory response and alleviates clinical symptoms of arthritis. Immunol. Lett. 2022, 242, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, X.; Hu, M.; Jia, M.; Jin, R.; Nie, Y. Recent progress in therapeutic strategies and biomimetic nanomedicines for rheumatoid arthritis treatment. Expert Opin. Drug Deliv. 2022, 19, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials 2022, 15, 1421. [Google Scholar] [CrossRef]
- Verdes, M.; Mace, K.; Margetts, L.; Cartmell, S. Status and challenges of electrical stimulation use in chronic wound healing. Curr. Opin. Biotechnol. 2022, 75, 102710. [Google Scholar] [CrossRef]
- Popa, M.; Atanase, L.I. Biological macromolecules for drug delivery in tissue engineering. In Biological Macromolecules; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–418. [Google Scholar] [CrossRef]
- Seppen, B.; Wiegel, J.; Ter Wee, M.; Schaardenburg, D.; Roorda, L.; Nurmohamed, M.; Boers, M.; Bos, W. Smartphone-Assisted Patient-Initiated Care Versus Usual care in Patients With Rheumatoid Arthritis and Low Disease Activity: A Randomized Controlled Trial. Arthritis Rheumatol. 2022, 1–9. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzym. Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Ding, Q.; Shiltz, D.; Hossami, D.; Konieczny, A.M. The economic burden of biologic disease-modifying antirheumatic drugs in rheumatoid arthritis patients in the United States. Expert Rev. Pharm. Outcomes Res. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choe, Y.; Lee, S. Lessons From the Success and Failure of Targeted Drugs for Rheumatoid Arthritis: Perspectives for Effective Basic and Translational Research. Immune Netw. 2022, 22, e8. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Brull, R.; Speer, J.; Hu, L.-Q.; Sawyer, T.; McCartney, C.J.L.; Abdallah, F.W. Analgesic benefits of the quadratus lumborum block in total hip arthroplasty: A systematic review and meta-analysis. Anaesthesia 2022, 77, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Plunkett, A.; Vijjeswarapu, A.M.; Hackworth, R.; Dhanjal, S.; Turan, A.; Cohen, S.P.; Eisenach, J.C.; Griffith, S.; Hanling, S.; et al. Percutaneous Neuromodulation of the Brachial Plexus and Sciatic Nerve for the Treatment of Acute Pain Following Surgery: Secondary Outcomes From a Multicenter, Randomized, Controlled Pilot Study. Neuromodul. Technol. Neural Interface 2022. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puerta, J.A.; Lobo-Prat, D.; Perez-García, C.; Ponce, A.; Frade-sosa, B.; Millán Arciniegas, A.M.; Ojeda, F.; Ruiz-Esquide, V.; Corominas, H. Clinical Patterns and Follow-Up of Inflammatory Arthritis and Other Immune-Related Adverse Events Induced by Checkpoint Inhibitors. A Multicenter Study. Front. Med. 2022, 9, 888377. [Google Scholar] [CrossRef]
- Ostoich, P.; Beltcheva, M.; Antonio Heredia Rojas, J.; Metcheva, R. Radionuclide Contamination as a Risk Factor in Terrestrial Ecosystems: Occurrence, Biological Risk, and Strategies for Remediation and Detoxification. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Rydell, N.M.D.; Balazs, E.A.M.D. Effect of Intra-articular Injection of Hyaluronic Acid on the Clinical Symptoms of Osteoarthritis and on Granulation Tissue Formation. Clin. Orthop. Relat. Res. 1971, 80, 25–32. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, S.W.; Kim, S.H.; Cardinal, J.R.; Jacobs, H. Drug release from hydrogel devices with ratecontrolling barriers. J. Membr. Sci. 1980, 7, 293–303. [Google Scholar] [CrossRef]
- Helme, R.D.; Koschorke, G.M.; Zimmermann, M. Immunoreactive substance P release from skin nerves in the rat by noxious thermal stimulation. Neurosci. Lett. 1986, 63, 295–299. [Google Scholar] [CrossRef]
- Bonanomi, M.H.; Velvart, M.; Stimpel, M.; Roos, K.M.; Fehr, K.; Weder, H.G. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatol. Int. 1987, 7, 203–212. [Google Scholar] [CrossRef]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, E.; McQueen, D.S.; Chessell, I.P.; Humphrey, P.P.A. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br. J. Pharmacol. 1998, 125, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vouldoukis, I.; Sivan, V.; Vozenin, M.C.; Kamaté, C.; Calenda, A.; Mazier, D.; Dugas, B. Fc-Receptor-Mediated Intracellular Delivery of Cu/Zn-superoxide Dismutase (SOD1) Protects Against Redox-Induced Apoptosis Through a Nitric Oxide Dependent Mechanism. Mol. Med. 2000, 6, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, A.G.; Wong, C.R.; Bawendi, M.G.; Frank, E.H.; Grodzinsky, A.J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 2014, 35, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Chow, G.M. Synthesis of nir-sensitive Au–Au2S nanocolloids for drug delivery. Mater. Sci. Eng. C 2003, 23, 113–116. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Sa, B. Electroresponsive Polyacrylamide-grafted-xanthan Hydrogels for Drug Delivery. J. Bioact. Compat. Polym. 2009, 24, 368–384. [Google Scholar] [CrossRef]
- Lv, Y.; Fang, M.; Zheng, J.; Yang, B.; Li, H.; Xiuzigao, Z.; Song, W.; Chen, Y.; Cao, W. Low-intensity Ultrasound Combined with 5-aminolevulinic Acid Administration in the Treatment of Human Tongue Squamous Carcinoma. Cell. Physiol. Biochem. 2012, 30, 321–333. [Google Scholar] [CrossRef]
- Mohammad, F.; Yusof, N.A. Doxorubicin-loaded magnetic gold nanoshells for a combination therapy of hyperthermia and drug delivery. J. Colloid Interface Sci. 2014, 434, 89–97. [Google Scholar] [CrossRef]
- Ricotti, L.; das Neves, R.P.; Ciofani, G.; Canale, C.; Nitti, S.; Mattoli, V.; Mazzolai, B.; Ferreira, L.; Menciassi, A. Boron nitride nanotube-mediated stimulation modulates F/G-actin ratio and mechanical properties of human dermal fibroblasts. J. Nanopart. Res. 2014, 16, 2247. [Google Scholar] [CrossRef]
- Ehrhardt, S.; Appel, L.J.; Meinert, C.L. Trends in National Institutes of Health Funding for Clinical Trials Registered in ClinicalTrials.gov. JAMA 2015, 314, 2566. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 157, e069722. [Google Scholar] [CrossRef]
- Hurysz, B.; Bottini, N. Emerging proteoglycans and proteoglycan-targeted therapies in rheumatoid arthritis. Am. J. Physiol.-Cell Physiol. 2022, 322, C1061–C1067. [Google Scholar] [CrossRef] [PubMed]
- Moukengue, B.; Lallier, M.; Marchandet, L.; Baud’huin, M.; Verrecchia, F.; Ory, B.; Lamoureux, F. Origin and Therapies of Osteosarcoma. Cancers 2022, 14, 3503. [Google Scholar] [CrossRef] [PubMed]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Tengvall, P.; Amengual-Peñafiel, L.; Coli, P.; Kotsakis, G.; Cochran, D.L. Implications of considering peri-implant bone loss a disease, a narrative review. Clin. Implant. Dent. Relat. Res. 2022, 24, 532–543. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; Fernandes, P.O.; Gonçalves, J.E.; Maltarollo, V.G.; Honorio, K.M. Recent Advances in the Prediction of Pharmacokinetics Properties in Drug Design Studies: A Review. ChemMedChem 2022, 17, 1–13. [Google Scholar] [CrossRef]
- Held, M.B.; Gazgalis, A.; Neuwirth, A.L.; Shah, R.P.; Cooper, H.J.; Geller, J.A. Imageless robotic-assisted total knee arthroplasty leads to similar 24-month WOMAC scores as compared to conventional total knee arthroplasty: A retrospective cohort study. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 30, 2631–2638. [Google Scholar] [CrossRef]
- Atkinson, A.J. Clinical pharmacokinetics. In Atkinson’s Principles of Clinical Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 11–26. [Google Scholar] [CrossRef]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Rivellese, F.; Surace, A.E.A.; Goldmann, K.; Sciacca, E.; Çubuk, C.; Giorli, G.; John, C.R.; Nerviani, A.; Fossati-Jimack, L.; Thorborn, G.; et al. Rituximab versus tocilizumab in rheumatoid arthritis: Synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat. Med. 2022, 28, 1256–1268. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Zhao, L.; Cheng, Y.; Gao, M.; Huang, C.; Wang, Z.; Wang, F. Tocilizumab in rheumatoid arthritis-associated peripheral ulcerative keratitis: A 1-year follow-up case report. Rheumatol. Autoimmun. 2022, 2, 45–50. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M.; et al. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J. Dermatol. Treat. 2022, 33, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, R.; Peng, L.; Liu, M.; Sun, Z. Efficacy and Safety of Adalimumab Biosimilars: Current Critical Clinical Data in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 638444. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Nair, N.; Plant, D.; Hyrich, K.; Morgan, A.W.; Isaacs, J.; Wilson, A.G.; Barton, A. P189 A longitudinal study of psychological predictors of response to adalimumab in patients with rheumatoid arthritis. Rheumatology 2022, 61, keac133.188. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Ganjoo, K.N.; Van Tine, B.A.; Riedel, R.F.; Chugh, R.; Cranmer, L.D.; Gordon, E.M.; Hornick, J.L.; Kwiatkowski, D.J.; et al. ABI-009 (nab-sirolimus) in advanced malignant perivascular epithelioid cell tumors (PEComa): Preliminary efficacy, safety, and mutational status from AMPECT, an open label phase II registration trial. J. Clin. Oncol. 2019, 37 (Suppl. S15), 11005. [Google Scholar] [CrossRef]
- Fala, L. Sivextro (Tedizolid Phosphate) Approved for the Treatment of Adults with Acute Bacterial Skin and Skin-Structure Infections. Am. Health Drug Benefits 2015, 8, 111–115. [Google Scholar] [PubMed]
- Tavakoli, M.; Mirhaj, M.; Labbaf, S.; Varshosaz, J.; Taymori, S.; Jafarpour, F.; Salehi, S.; Abadi, S.A.M.; Sepyani, A. Fabrication and evaluation of Cs/PVP sponge containing platelet-rich fibrin as a wound healing accelerator: An in vitro and in vivo study. Int. J. Biol. Macromol. 2022, 204, 245–257. [Google Scholar] [CrossRef]
- Li, M.; Vora, L.K.; Peng, K.; Donnelly, R.F. Trilayer microneedle array assisted transdermal and intradermal delivery of dexamethasone. Int. J. Pharm. 2022, 612, 121295. [Google Scholar] [CrossRef]
- Kubo, T.; Kumai, T.; Ikegami, H.; Kano, K.; Nishii, M.; Seo, T. Diclofenac–hyaluronate conjugate (diclofenac etalhyaluronate) intra-articular injection for hip, ankle, shoulder, and elbow osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 371. [Google Scholar] [CrossRef]
- Branco, A.C.; Oliveira, A.S.; Monteiro, I.; Nolasco, P.; Silva, D.C.; Figueiredo-Pina, C.G.; Colaço, R.; Serro, A.P. PVA-Based Hydrogels Loaded with Diclofenac for Cartilage Replacement. Gels 2022, 8, 143. [Google Scholar] [CrossRef]
- Kumar, V.; Sethi, B.; Yanez, E.; Leung, D.H.; Ghanwatkar, Y.Y.; Cheong, J.; Tso, J.; Narang, A.S.; Nagapudi, K.; Mahato, R.I. Effect of magnesium stearate surface coating method on the aerosol performance and permeability of micronized fluticasone propionate. Int. J. Pharm. 2022, 615, 121470. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, J.H.; Kim, M.W.; Kim, S.H.; Yong, S.Y. Study of the Efficacy of Artificial Intelligence Algorithm-Based Analysis of the Functional and Anatomical Improvement in Polynucleotide Treatment in Knee Osteoarthritis Patients: A Prospective Case Series. J. Clin. Med. 2022, 11, 2845. [Google Scholar] [CrossRef] [PubMed]
- Hummer, C.D.; Huang, Y.; Sheehan, B. Adherence to the OARSI recommendations for designing, conducting, and reporting of clinical trials in knee osteoarthritis: A targeted literature review. BMC Musculoskelet. Disord. 2022, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.M.; Baker, J. Treatment of early rheumatoid arthritis: Methotrexate and beyond. Curr. Opin. Pharmacol. 2022, 64, 102227. [Google Scholar] [CrossRef] [PubMed]
- Giollo, A.; Fuzzi, E.; Doria, A. Methotrexate in early rheumatoid arthritis: Is the anchor drug still holding? Autoimmun. Rev. 2022, 21, 103031. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef]
- Mittal, N.; Seyboth, B.; Kent, P. Checkpoint inhibitor and multireceptor tyrosine kinase inhibitor combination in relapsed refractory sarcomas: A single institution series. J. Clin. Oncol. 2022, 40 (Suppl. S16), 11529. [Google Scholar] [CrossRef]
- Wen, X.; Pan, Q.; Xu, B.; Xiao, W.; Weng, D.; Zhao, J.; Xu, H.; Huang, Z.; Niu, X.; Zhang, X. Phase I study of pegylated liposomal doxorubicin and cisplatin in patients with advanced osteosarcoma. Cancer Chemother. Pharmacol. 2022, 89, 209–215. [Google Scholar] [CrossRef]
- Huang, G.; Hua, S.; Liu, H.; Zhou, H.; Chen, X.; Wang, Z.; Yu, W. Efficacy of ifosfamide combined with liposome doxorubicin on osteosarcoma and its effects on serum IL-10, TNF-α, and IFN-γ in patients with osteosarcoma. Am. J. Transl. Res. 2022, 14, 1288–1296. [Google Scholar]
- Iqbal, K.; Milioudi, A.; Wicha, S.G. Pharmacokinetics and Pharmacodynamics of Tedizolid. Clin. Pharmacokinet. 2022, 61, 489–503. [Google Scholar] [CrossRef]
- Jorda, A.; Wulkersdorfer, B.; Schörgenhofer, C.; Matzneller, P.; Al Jalali, V.; Bauer, M.; Wölf-Duchek, M.; Lackner, E.; Dorn, C.; Jilma, B.; et al. Influence of tedizolid on the cytokine response to the endotoxin challenge in healthy volunteers: A cross-over trial. J. Antimicrob. Chemother. 2022, 77, 1424–1431. [Google Scholar] [CrossRef]
- García-Sobrino, R.; Casado-Losada, I.; Bruno-Pérez, L.; García, C.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A.; Martínez-Campos, E. Thermosensitive hydrogels functionalized with pH sensitive COOH groups for bone cell harvesting. Eur. Polym. J. 2022, 169, 111131. [Google Scholar] [CrossRef]
- Diniz, J.A.; Barbirato, D.D.S.; do Nascimento, E.H.L.; dos Anjos Pontual, A.; Dourado, A.C.A.G.; Laureano Filho, J.R. Tomographic evaluation of the effect of simvastatin topical use on alveolar bone microarchitecture, pain and swelling after mandibular third molar extraction: A randomized controlled trial. Clin. Oral Investig. 2022, 26, 3533–3545. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Chang, C.-C.; Wei, J.C.-C.; Lin, H.-Y.; Brown, C.; Tai, T.-T.; Wu, C.-F.; Chuang, W.C.-M.; Shih, S.-F. TLC599 in patients with osteoarthritis of the knee: A phase IIa, randomized, placebo-controlled, dose-finding study. Arthritis Res. Ther. 2022, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Wu, C.-F.; Chuang, W.; Shih, S.-F. Single intra-articular injection of TLC599 in patients with osteoarthritis knee pain: Subgroup analyses of a placebo-controlled 24-week phase 2 trial. Osteoarthr. Cartil. 2020, 28, S481–S482. [Google Scholar] [CrossRef]
- Kisukeda, T.; Onaya, J.; Yoshioka, K. Effect of diclofenac etalhyaluronate (SI-613) on the production of high molecular weight sodium hyaluronate in human synoviocytes. BMC Musculoskelet. Disord. 2019, 20, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, Y.; Kano, K.; Osato, T.; Seo, T. Open-label phase 3 study of diclofenac conjugated to hyaluronate (diclofenac etalhyaluronate: ONO-5704/SI-613) for treatment of osteoarthritis: 1-year follow-up. BMC Musculoskelet. Disord. 2021, 22, 233. [Google Scholar] [CrossRef]
- Malone, A.; Price, J.; Price, N.; Peck, V.; Getgood, A.; Petrella, R.; Helliwell, J. Safety and pharmacokinetics of EP-104IAR (sustained-release fluticasone propionate) in knee osteoarthritis: A randomized, double-blind, placebo-controlled phase 1 trial. Osteoarthr. Cartil. Open 2021, 3, 100213. [Google Scholar] [CrossRef]
- Getgood, A.; Dhollander, A.; Malone, A.; Price, J.; Helliwell, J. Pharmacokinetic Profile of Intra-articular Fluticasone Propionate Microparticles in Beagle Dog Knees. CARTILAGE 2017, 10, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; Del Piccolo, N.; Rani, N.; Govoni, M.; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, double-blind comparison of a fixed co-formulation of intra-articular polynucleotides and hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: Two-year follow-up. BMC Musculoskelet. Disord. 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, T.-R.; Lee, S.E.; Na Jang, Y.; Han, H.S.; Mun, S.K.; Kim, B.J. Comparative Evaluation of the Effectiveness of Novel Hyaluronic Acid-Polynucleotide Complex Dermal Filler. Sci. Rep. 2020, 10, 5127. [Google Scholar] [CrossRef] [Green Version]
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Baroni, S.S.; Pavani, M.; Moretti, M.; et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2020, 193, 111413. [Google Scholar] [CrossRef]
- Zisman, D.; Safieh, M.; Simanovich, E.; Feld, J.; Kinarty, A.; Zisman, L.; Gazitt, T.; Haddad, A.; Elias, M.; Rosner, I.; et al. Tocilizumab (TCZ) Decreases Angiogenesis in Rheumatoid Arthritis Through Its Regulatory Effect on miR-146a-5p and EMMPRIN/CD147. Front. Immunol. 2021, 12, 739592. [Google Scholar] [CrossRef]
- Coates, L.C.; Tillett, W.; D’Agostino, M.-A.; Rahman, P.; Behrens, F.; McDearmon-Blondell, E.L.; Bu, X.; Chen, L.; Kapoor, M.; Conaghan, P.G.; et al. Comparison between adalimumab introduction and methotrexate dose escalation in patients with inadequately controlled psoriatic arthritis (CONTROL): A randomised, open-label, two-part, phase 4 study. Lancet Rheumatol. 2022, 4, e262–e273. [Google Scholar] [CrossRef]
- Burmester, G.-R.; Kivitz, A.J.; Kupper, H.; Arulmani, U.; Florentinus, S.; Goss, S.L.; Rathmann, S.S.; Fleischmann, R.M. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: The randomised CONCERTO trial. Ann. Rheum. Dis. 2015, 74, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.M.; Chua-Alcala, V.S.; Kim, K.; Baby, R.; Angel, N.; Quon, D.; Wong, S.; Chawla, S.P. A phase I/II investigation of nivolumab and ABI-009 (nab-sirolimus) in advanced undifferentiated pleomorphic sarcoma (UPS), liposarcoma (LPS), chondrosarcoma (CS), osteosarcoma (OS), and Ewing sarcoma: Preliminary efficacy and safety results. J. Clin. Oncol. 2019, 37 (Suppl. S15), 11057. [Google Scholar] [CrossRef]
- Hou, S.; Schmid, A.; Desai, N. Abstract 348: ABI-009 (nab-Sirolimus) improves tumor accumulation and antitumor activity over oral mTOR inhibitors. Exp. Mol. Ther. 2019, 79, 348. [Google Scholar] [CrossRef]
- Huang, S.; Du, K.; Liu, Z.; Li, J. Inhibition of mTOR by temsirolimus overcomes radio-resistance in nasopharyngeal carcinoma. Clin. Exp. Pharmacol. Physiol. 2022, 49, 703–709. [Google Scholar] [CrossRef]
- Laukkanen, S.; Veloso, A.B.; Yan, C.; Oksa, L.; Alpert, E.J.; Do, D.; Hyvärinen, N.; McCarthy, K.; Adhikari, A.; Yang, Q.; et al. Combination therapies to inhibit LCK tyrosine kinase and mTOR signaling in T-cell Acute Lymphoblastic Leukemia. Blood 2022, 140. [Google Scholar] [CrossRef]
- Wong, E.; Rab, S. Tedizolid phosphate (sivextro): A second-generation oxazolidinone to treat acute bacterial skin and skin structure infections. Pharm. Ther. 2014, 39, 555–579. [Google Scholar]
- Vashistha, V.K.; Verma, N.; Kumar, R.; Tyagi, I.; Gaur, A.; Bala, R. Enantioseparation of linezolid and tedizolid using validated high-performance liquid chromatographic method. Chirality 2022, 34, 1044–1052. [Google Scholar] [CrossRef]
- Raafat, S.N.; Amin, R.M.; Elmazar, M.M.; Khattab, M.M.; El-Khatib, A.S. The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone 2018, 117, 60–69. [Google Scholar] [CrossRef]
- Gupta, S.; Verma, P.; Tikku, A.P.; Chandra, A.; Yadav, R.K.; Bharti, R.; Bains, R. “Effect of local application of simvastatin in bone regeneration of peri-apical defects-a clinico-radiographic study. J. Oral Biol. Craniofac. Res. 2020, 10, 583–591. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Gremese, E.; Tolusso, B.; Bruno, D.; Alivernini, S.; Ferraccioli, G. Infectious agents breaking the immunological tolerance: The holy grail in rheumatoid arthritis reconsidered. Autoimmun. Rev. 2022, 21, 103102. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, S.; Lv, X.; Gan, Y.; Luo, Y.; Li, T. Paediatric Osteomyelitis and Septic Arthritis Pathogen Distribution and Antimicrobial Resistance in a Single Centre: A 15-Year Retrospective Analysis. J. Trop. Pediatr. 2022, 68, fmac038. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-Based Composite Materials Used in Drug Delivery Systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Yang, M.; Xu, X. Important roles of transporters in the pharmacokinetics of anti-viral nucleoside/nucleotide analogs. Expert Opin. Drug Metab. Toxicol. 2022, 18, 483–505. [Google Scholar] [CrossRef]
- Agrawal, P.; Nikhade, P.; Chandak, M.; Ikhar, A.; Bhonde, R. Dentin Matrix Metalloproteinases: A Futuristic Approach Toward Dentin Repair and Regeneration. Cureus 2022, 14, e27946. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Tian, K.; Du, G.; Wang, X.; Wu, X.; Li, L.; Liu, W.; Wu, R. MMP-9 secreted by M2-type macrophages promotes Wilms’ tumour metastasis through the PI3K/AKT pathway. Mol. Biol. Rep. 2022, 49, 3469–3480. [Google Scholar] [CrossRef]
- Kilic, T.; Okuno, K.; Eguchi, S.; Kassiri, Z. Disintegrin and Metalloproteinases (ADAMs [A Disintegrin and Metalloproteinase] and ADAMTSs [ADAMs With a Thrombospondin Motif]) in Aortic Aneurysm. Hypertension 2022, 79, 1327–1338. [Google Scholar] [CrossRef]
- Kirman, D.C.; Renganathan, B.; Chui, W.K.; Chen, M.W.; Kaya, N.A.; Ge, R. Cell surface nucleolin is a novel ADAMTS5 receptor mediating endothelial cell apoptosis. Cell Death Dis. 2022, 13, 172. [Google Scholar] [CrossRef]
- Scott, K.M.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. miR-122 and the WNT/β-catenin pathway inhibit effects of both interleukin-1β and tumor necrosis factor-α in articular chondrocytes in vitro. J. Cell. Biochem. 2022, 123, 1053–1063. [Google Scholar] [CrossRef]
- Joshi, N.; Yan, J.; Levy, S.; Bhagchandani, S.; Slaughter, K.V.; Sherman, N.E.; Amirault, J.; Wang, Y.; Riegel, L.; He, X.; et al. Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Q.; Velagapudi, R.; Barclay, W.E.; Rodriguiz, R.M.; Wetsel, W.C.; Yang, T.; Shinohara, M.L.; Terrando, N. Annexin-A1 Tripeptide Attenuates Surgery-Induced Neuroinflammation and Memory Deficits Through Regulation the NLRP3 Inflammasome. Front. Immunol. 2022, 13, 856254. [Google Scholar] [CrossRef]
- Li, N.; Qiao, Y.; Xue, L.; Xu, S.; Zhang, N. Targeted and MMP-2/9 responsive peptides for the treatment of rheumatoid arthritis. Int. J. Pharm. 2019, 569, 118625. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Q.; Zhang, Z.; Shi, K.; Sun, Y.; Liu, T.; Lin, J.; Yang, K. Albumin mediated reactive oxygen species scavenging and targeted delivery of methotrexate for rheumatoid arthritis therapy. Nano Res. 2021, 15, 153–161. [Google Scholar] [CrossRef]
- Shen, Q.; Hu, Q.; Tang, T.; Ying, X.; Shu, G.; Shen, J.; Teng, C.; Du, Y. ICAM-1 targeted thermal-sensitive micelles loaded with tofacitinib for enhanced treatment of rheumatoid arthritis via microwave assistance. Biomater. Adv. 2022, 138, 212940. [Google Scholar] [CrossRef]
- Lee, H.-S.; Dastgheyb, S.S.; Hickok, N.J.; Eckmann, D.M.; Composto, R.J. Targeted Release of Tobramycin from a pH-Responsive Grafted Bilayer Challenged with S. aureus. Biomacromolecules 2015, 16, 650–659. [Google Scholar] [CrossRef]
- Wang, X.; Cao, W.; Sun, C.; Wang, Y.; Wang, M.; Wu, J. Development of pH-sensitive dextran-based methotrexate nanodrug for rheumatoid arthritis therapy through inhibition of JAK-STAT pathways. Int. J. Pharm. 2022, 622, 121874. [Google Scholar] [CrossRef]
- Funk, R.H. Biophysical mechanisms complementing ldquo classical rdquo cell biology. Front. Biosci. 2018, 23, 4625. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, R.; Weinstein, R.C. Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986, 123, 377–401. [Google Scholar] [CrossRef]
- Chernet, B.T.; Levin, M. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis. Model. Mech. 2013, 6, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Bátai, I.Z.; Dombi, Á.; Borbély, É.; Fehér, Á.; Papp, F.; Varga, Z.; Mócsai, A.; Helyes, Z.; Pintér, E.; Pozsgai, G. Investigation of the Role of the TRPA1 Ion Channel in Conveying the Effect of Dimethyl Trisulfide on Vascular and Histological Changes in Serum-Transfer Arthritis. Pharmaceuticals 2022, 15, 671. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Dai, Y.; Ma, P.; Li, X.; Cheng, Z.; Hou, Z.; Kang, X.; Li, C.; Lin, J. Multifunctional Up-Converting Nanocomposites with Smart Polymer Brushes Gated Mesopores for Cell Imaging and Thermo/pH Dual-Responsive Drug Controlled Release. Adv. Funct. Mater. 2013, 23, 4067–4078. [Google Scholar] [CrossRef]
- Wen, K.; Zhou, M.; Lu, H.; Bi, Y.; Ruan, L.; Chen, J.; Hu, Y. Near-Infrared/pH Dual-Sensitive Nanocarriers for Enhanced Intracellular Delivery of Doxorubicin. ACS Biomater. Sci. Eng. 2018, 4, 4244–4254. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Song, H.; Zhang, L. Targeted Delivery System Based on Magnetic Mesoporous Silica Nanocomposites with Light-Controlled Release Character. ACS Appl. Mater. Interfaces 2012, 5, 11–15. [Google Scholar] [CrossRef]
- Yu, Y.-T.; Shi, S.-W.; Wang, Y.; Zhang, Q.-L.; Gao, S.-H.; Yang, S.-P.; Liu, J.-G. A Ruthenium Nitrosyl-Functionalized Magnetic Nanoplatform with Near-Infrared Light-Controlled Nitric Oxide Delivery and Photothermal Effect for Enhanced Antitumor and Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 312–321. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, C.; Chen, X.; Liu, J.; Yu, Q.; Liu, Y.; Liu, J. Drug Delivery System Based on Near-Infrared Light-Responsive Molybdenum Disulfide Nanosheets Controls the High-Efficiency Release of Dexamethasone To Inhibit Inflammation and Treat Osteoarthritis. ACS Appl. Mater. Interfaces 2019, 11, 11587–11601. [Google Scholar] [CrossRef]
- Kim, J.; Yu, A.M.; Kubelick, K.P.; Emelianov, S.Y. Gold nanoparticles conjugated with DNA aptamer for photoacoustic detection of human matrix metalloproteinase-9. Photoacoustics 2022, 25, 100307. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N.M. Hybrid Iron Oxide–Graphene Oxide–Polysaccharides Microcapsule: A Micro-Matryoshka for On-Demand Drug Release and Antitumor Therapy In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Je, J.-Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef]
- Jia, C.; Wu, H.; Luo, K.; Hao, W.; Wang, S.; Huang, M. Magnetic Silica Nanosystems With NIR-Responsive and Redox Reaction Capacity for Drug Delivery and Tumor Therapy. Front. Chem. 2020, 8, 567652. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jin, W.; Zuo, X.; Ji, S.; Nan, W.; Chen, H.; Gao, S.; Zhang, Q. Construction of small-sized superparamagnetic Janus nanoparticles and their application in cancer combined chemotherapy and magnetic hyperthermia. Biomater. Sci. 2020, 8, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1571. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Huang, X.; Li, F.; Davis, T.P.; Qiao, R.; Ling, D. Polymer-Assisted Magnetic Nanoparticle Assemblies for Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Hodgins, P.; Peterson, G.P. Experimental study of fundamental mechanisms in inductive heating of ferromagnetic nanoparticles suspension (Fe3O4 Iron Oxide Ferrofluid). J. Appl. Phys. 2011, 110, 054303. [Google Scholar] [CrossRef]
- Néel, L. Théorie du traînage magnétique des substances massives dans le domaine de Rayleigh. J. Phys. 1950, 11, 49–61. [Google Scholar] [CrossRef]
- Brown, W.F. Thermal Fluctuations of a Single-Domain Particle. Phys. Rev. 1963, 130, 1677–1686. [Google Scholar] [CrossRef]
- Su, Y.-L.; Fang, J.-H.; Liao, C.-Y.; Lin, C.-T.; Li, Y.-T.; Hu, S.-H. Targeted Mesoporous Iron Oxide Nanoparticles-Encapsulated Perfluorohexane and a Hydrophobic Drug for Deep Tumor Penetration and Therapy. Theranostics 2015, 5, 1233–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modak, M.; Bobbala, S.; Lescott, C.; Liu, Y.-G.; Nandwana, V.; Dravid, V.P.; Scott, E.A. Magnetic Nanostructure-Loaded Bicontinuous Nanospheres Support Multicargo Intracellular Delivery and Oxidation-Responsive Morphological Transitions. ACS Appl. Mater. Interfaces 2020, 12, 55584–55595. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Mahdavinia, G.R.; Kazemi, B.; Ehrlich, H.; Joseph, Y.; Rahimi-Nasrabadi, M. Highly efficient sunitinib release from pH-responsive mHPMC@Chitosan core-shell nanoparticles. Carbohydr. Polym. 2021, 258, 117719. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Arkan, E.; Derakhshankhah, H.; Haghshenas, B.; Jahanban-Esfahlan, R.; Jaymand, M. A novel bioreducible and pH-responsive magnetic nanohydrogel based on β-cyclodextrin for chemo/hyperthermia therapy of cancer. Carbohydr. Polym. 2021, 252, 117229. [Google Scholar] [CrossRef]
- Jing, Y.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Ultrasound-Triggered Smart Drug Release from Multifunctional Core−Shell Capsules One-Step Fabricated by Coaxial Electrospray Method. Langmuir 2011, 27, 1175–1180. [Google Scholar] [CrossRef]
- Wang, X.; Yan, F.; Liu, X.; Wang, P.; Shao, S.; Sun, Y.; Sheng, Z.; Liu, Q.; Lovell, J.F.; Zheng, H. Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins. J. Control Release 2018, 286, 358–368. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, X.; Yang, H.; Zhang, S.; Li, Y.; Kuang, C.; Fu, S.; Cui, L.; Liang, M.; Gao, C.; et al. Novel Class of Ultrasound-Triggerable Drug Delivery Systems for the Improved Treatment of Tumors. Mol. Pharm. 2019, 16, 2956–2965. [Google Scholar] [CrossRef]
- Dwivedi, P.; Kiran, S.; Han, S.; Dwivedi, M.; Khatik, R.; Fan, R.; Mangrio, F.A.; Du, K.; Zhu, Z.; Yang, C.; et al. Magnetic Targeting and Ultrasound Activation of Liposome–Microbubble Conjugate for Enhanced Delivery of Anticancer Therapies. ACS Appl. Mater. Interfaces 2020, 12, 23737–23751. [Google Scholar] [CrossRef]
- Wu, P.; Dong, W.; Guo, X.; Qiao, X.; Guo, S.; Zhang, L.; Wan, M.; Zong, Y. ROS-Responsive Blended Nanoparticles: Cascade-Amplifying Synergistic Effects of Sonochemotherapy with On-demand Boosted Drug Release During SDT Process. Adv. Health Mater. 2019, 8, 1900720. [Google Scholar] [CrossRef]
- Kim, S.; Im, S.; Park, E.-Y.; Lee, J.; Kim, C.; Kim, T.-I.; Kim, W.J. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102110. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Chang, Y.; Sun, C.; Shao, S.; Xie, Z.; Xiao, X.; Ma, P.; Zhang, H.; Cheng, Z.; et al. Intelligent Hollow Pt-CuS Janus Architecture for Synergistic Catalysis-Enhanced Sonodynamic and Photothermal Cancer Therapy. Nano Lett. 2019, 19, 4134–4145. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Wells, C.M.; Jennings, J.A.; Ghimire, M.; Mishra, S.R.; Morshed, B.I. Electric Stimulus-Responsive Chitosan/MNP Composite Microbeads for a Drug Delivery System. IEEE Trans. Biomed. Eng. 2020, 67, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Dao, D.V.; Mitsubayashi, K. Biosensors and Chemical Sensors for Healthcare Monitoring: A Review. IEEJ Trans. Electr. Electron. Eng. 2022, 17, 626–636. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials 2022, 15, 7672. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Ahmad, M.M.H.M.; Abu Bakar, M. Numerical Simulation Study on Relationship between the Fracture Mechanisms and Residual Membrane Stresses of Metallic Material. J. Funct. Biomater. 2022, 13, 20. [Google Scholar] [CrossRef]
- Forero, J.; Roa, E.; Reyes, J.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B Chem. 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Gharehdaghi, Z.; Rahimi, R.; Naghib, S.M.; Molaabasi, F. Fabrication and application of copper metal–organic frameworks as nanocarriers for pH-responsive anticancer drug delivery. J. Iran. Chem. Soc. 2022, 19, 2727–2737. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Mao, Y.; Li, Z. Self-Powerbility in Electrical Stimulation Drug Delivery System. Adv. Mater. Technol. 2022, 7, 2100055. [Google Scholar] [CrossRef]
- Atoufi, Z.; Zarintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/ agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, W.; Kwak, K.; Choi, H.; Kim, D.-H. Electric Pulse Responsive Magnetic Nanoclusters Loaded with Indoleamine 2,3-Dioxygenase Inhibitor for Synergistic Immuno-Ablation Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54415–54425. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Inamdar, S.Z.; Das, K.K.; Akamanchi, K.G.; Patil, A.V.; Inamadar, A.C.; Reddy, K.R.; Raghu, A.V.; Kulkarni, R.V. Tailor-made electrically-responsive poly(acrylamide)-graft-pullulan copolymer based transdermal drug delivery systems: Synthesis, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101525. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Liu, H.; Li, Z.; Sun, Q.; Wang, Y.; Fan, X. A sensitive electrochemiluminescence biosensor for assay of cancer biomarker (MMP-2) based on NGQDs-Ru@SiO2 luminophore. Talanta 2021, 236, 122830. [Google Scholar] [CrossRef]
- Rowsell, S.; Hawtin, P.; Minshull, C.A.; Jepson, H.; Brockbank, S.M.; Barratt, D.G.; Slater, A.M.; McPheat, W.L.; Waterson, D.; Henney, A.M.; et al. Crystal Structure of Human MMP9 in Complex with a Reverse Hydroxamate Inhibitor. J. Mol. Biol. 2002, 319, 173–181. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Han, H. A novel impedance enhancer for amperometric biosensor based ultrasensitive detection of matrix metalloproteinase-2. Bioelectrochemistry 2019, 130, 107324. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Xu, Q.; Hu, J.; Zhang, C.-Y. Construction of a sensitive protease sensor with DNA-peptide conjugates for single-molecule detection of multiple matrix metalloproteinases. Biosens. Bioelectron. 2020, 169, 112647. [Google Scholar] [CrossRef]
- Cheng, W.; Ma, J.; Kong, D.; Zhang, Z.; Khan, A.; Yi, C.; Hu, K.; Yi, Y.; Li, J. One step electrochemical detection for matrix metalloproteinase 2 based on anodic stripping of silver nanoparticles mediated by host-guest interactions. Sens. Actuators B Chem. 2021, 330, 129379. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.; Jeong, S.H.; Lee, E.; Lee, W.; Liu, N.; Yoon, D.S.; Kim, S.; Lee, S.W. MoS2 Field-Effect Transistor-Amyloid-β1–42 Hybrid Device for Signal Amplified Detection of MMP-9. Anal. Chem. 2019, 91, 8252–8258. [Google Scholar] [CrossRef]
- Su, H.; Zang, M.; Lu, L.; Li, F. Monitoring matrix metalloproteases based on the selective interaction between an Ir(iii) solvent complex and a histidine-rich polypeptide. Chem. Commun. 2019, 55, 7085–7088. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-Shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Viennois, E.; Fang, J.; Merlin, D.; Iyer, S.S. Toward Point-of-Care Diagnostics to Monitor MMP-9 and TNF-α Levels in Inflammatory Bowel Disease. ACS Omega 2021, 6, 6582–6587. [Google Scholar] [CrossRef] [PubMed]
- Nisiewicz, M.K.; Kowalczyk, A.; Gajda, A.; Kasprzak, A.; Bamburowicz-Klimkowska, M.; Grudzinski, I.P.; Nowicka, A.M. Enzymatic cleavage of specific dipeptide conjugated with ferrocene as a flexible ultra-sensitive and fast voltammetric assay of matrix metalloproteinase-9 considered a prognostic cancer biomarker in plasma samples. Biosens. Bioelectron. 2022, 195, 113653. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, S.; Pauptit, R.A. MMP9-Inhibitor Complex. Available online: https://www.wwpdb.org/pdb?id=pdb_00001gkc (accessed on 21 September 2022).







| Drug Carrier | Arthritis Therapy | API | GCT I, Year | Primary Test | Refs. |
|---|---|---|---|---|---|
| TLC599 (BioSeizer®) | Knee OA | Dex sodium phosphate | NCT03754049, 2022 | Pharmacokinetics | [76,77] |
| SI-613 | Knee OA | Diclofenac (Voltaren®) | NCT03209362, 2021 | WOMAC | [78,79] |
| EP-104IAR | Knee OA | Fluticasone propionate | NCT02609126, 2021 | Pharmacokinetics | [80,81] |
| HA-PN (Condrotide® Plus) | Knee OA | PN | NCT02417610, 2017 | WOMAC | [82,83] |
| miR-146a | RA | Tocilizumab (Actemra®) | NCT03149796, 2017 | miR expressions | [84,85] |
| Adalimumab (Humira®) | RA | Mtx | NCT01185288, 2014 | Pharmacokinetics | [86,87] |
| ABI-009 | Osteosarcoma | Nivolumab (Opdivo®) | NCT03190174, 2021 | Pharmacokinetics | [88,89] |
| Temsirolimus (Torisel®) | Osteosarcoma | Liposomal Dox (Doxil®) | NCT00949325, 2019 | Pharmacokinetics | [90,91] |
| Ted phosphate (Sivextro®) | Joint infections | Ted | NCT03378427, 2021 | Immuno-compromised | [92,93] |
| PRF | Peri-implant bone | Simvastatin (Zocor®) | NCT05008068, 2021 | Bone regeneration | [94,95] |
| Biosensor | pH Stimulus | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|---|
| CuS | - | Dex | CuS@NR | High efficiency, target reachability, and long-circulated releases | [118] |
| UCNP | NIPAMAA | Dox | UCNP@SiO2/PIPAMAA | Release increased with higher temperature and lower pH values | [119] |
| UCNP | PBAE | Dox | PBAE@UCNP | Enhanced PBAE protonation in lysosomes to release Dox | [120] |
| Trigger | Assistance | Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Azo | magnetic | MMS | Ibuprofen | Azo-MMS | Used magnetic-aid targetor | [121] |
| Ru | magnetic | IONP | NO, PTT | IONP/PDA/Ru-NO/FA | Enhanced antitumor efficacy with targetor | [122] |
| MoS2 | PA | MoS2 | Dex | MoS2/Chi | Reduced cartilage erosion, TNF-α, and IL-1β secretions | [123] |
| Au | PA | DNAA | DMSO | Au1/DNAA/Au2 | Selective and sensitive detection of human MMP-9 | [124] |
| Stimuli | Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|---|
| pH | Chi | Dox | Alg/Chi/IONP@ GO/HA | Synchronised effect of thermal and chemotherapy | [125] |
| pH | DMSA | Dox | IONP/DMSA | NIR induced Fe3+ temperature excellently | [126] |
| Redox | PDA | Dox | IONP/SiO2@PDA | Additional redox stimulus from hydroxyl radicals | [127] |
| Stimuli | Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|---|
| Redox | PPS | Dox, camptothecin | PEG-block-PPS/IONP | Heat trigger for API inductions in multicargo intracellular DDS | [135] |
| pH | Chitosan | Sunitinib | HPMC/IONP-Chi | Remote release of sunitinib efficiently at pH 4.5 | [136] |
| Redox, pH | PEtOx | Dox | β-cyclodextrin/g-(PEtOx)7/IONP | Ionised tumours at pH 5 in chemo-hyperthermia cancer therapy | [137] |
| Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|
| TiO2 | Ptx | TiO2/IONP/PEG-QDs | US duration control drug release profile | [138] |
| Porphyrin | Dox | PP | Enhanced local delivery and tumour suppression | [139] |
| NGR | Dox | DSPE/PEG2k/NGR | Triggered lipid bilayer to break down in antitumor effects | [140] |
| PFCMB | Dox | IONP/PEG/PFCMB | Deeper site targeting and precise delivery | [141] |
| Trigger | Stimulus | Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|---|---|
| IR780 | Redox | TL | Ptx | DSPE-PEG2k-NH2/IR780/TL | US induced ROS for tumour growth inhibition and apoptosis | [142] |
| TiO2 | Redox | PBE | Dox | TiO2-PBE | High local accumulation and tumour growth inhibition | [143] |
| TAPP | Electro-ion | CuS and Pt | H2O2 | Pt-CuS/TAPP | Pt catalyses H2O2 for tumour hypoxia and cell apoptosis therapies | [144] |
| Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|
| CuZn | HAp | CuZn/G/Chi | Anti-bacterial activity increased, but osteoprogenitor cells were not cytotoxic | [150] |
| Cu MOF | miR-155 | Au@Cu MOF | Current response change of 25 µA and the DPV detection limit of 0.35 fM | [151] |
| Cu MOF | Dox | (BTC)2/Cu3/IONP | Adsorbed 40.5% and released 85.5% at pH 5 | [152] |
| Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|
| AT | Dex | Agr/Alg/AT | Enhanced cell viability and proliferation for neuroregenerative medicine | [154] |
| IONC | IDOi | IDOi/IONC | Synergistic effects on immuno-ablation cancer therapy with local magnetic field | [155] |
| PEGDMA | Vcm | Chi/IONP/PEGDMA | Controllable stimulation of DDS | [145] |
| Plt/PVA | RT | Plt/PVA/ PAA | Efficient transdermal DDS | [156] |
| Biosensor | Cleavage Site | Drug Carrier | Test Remarks | Ref. |
|---|---|---|---|---|
| MMP-2 | Gly-Val | CS-Au-Pb/P-NH2/PANI 1 | Electrochemical | [159] |
| MMP-2 | Gly-Val | DNA-P/rDNA 2 | Electrochemical | [160] |
| MMP-2 | Gly-Val | PMUA/CB8/Ag/P2 3 | Electrochemical | [161] |
| MMP-2 | Sulfhydryl P 4 | NGQDs/sulfhydryl P/Ru@SiO2 | Electrochemical | [157] |
| MMP-9 | Leu-Met | MoS2/Si/Aβ1–42 | Circulating protein by FET | [162] |
| MMP-9 | Gly-Leu | Fe3O4/Ir(III)His-P | Fe3O4 by magnetic, Ir(III) by FRET | [163] |
| MMP-2/-9 | Gly-Met | SiO2/rhodamine b/P-P 5 | Rhodamine b by FRET | [165] |
| MMP-9 | Gly-Met | Au/CSH/Gly-Met/ferrocene | DPV | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.Y.; Zaidi, A.M.A.; Miskon, A. Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy. Pharmaceuticals 2022, 15, 1330. https://doi.org/10.3390/ph15111330
Lim YY, Zaidi AMA, Miskon A. Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy. Pharmaceuticals. 2022; 15(11):1330. https://doi.org/10.3390/ph15111330
Chicago/Turabian StyleLim, Yan Yik, Ahmad Mujahid Ahmad Zaidi, and Azizi Miskon. 2022. "Composing On-Program Triggers and On-Demand Stimuli into Biosensor Drug Carriers in Drug Delivery Systems for Programmable Arthritis Therapy" Pharmaceuticals 15, no. 11: 1330. https://doi.org/10.3390/ph15111330