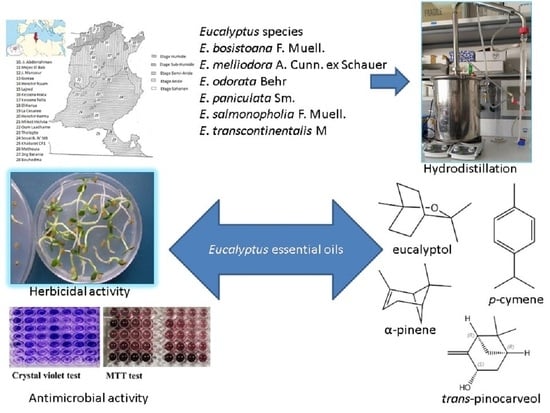
Chemistry and Bioactivities of Six Tunisian Eucalyptus Species
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Essentials Oils
2.2. Phytotoxic Activity
2.3. Antibiofilm Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Essential Oils
3.3. Analysis of Essential Oils
3.4. Phytotoxic Activity
3.5. Antibacterial Activity
3.5.1. Microorganisms and Culture Conditions
3.5.2. Minimal Inhibitory Concentration (MIC)
3.5.3. Biofilm Inhibitory Activity
3.5.4. Inhibition of the Bacterial Metabolism
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umereweneza, D.; Muhizi, T.; Kamizikunze, T.; Nkurunziza, J.P. Chemical Composition and Antifungal Activity of Essential Oils Extracted from Leaves of Eucalyptus melliodora and Eucalyptus anceps Grown in Rwanda. J. Essent. Oil Bear. Plants 2019, 22, 151–158. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Dorsaf, B.H.; Hanen, B.I.; Chokri, J.; Larbi Khouja, M.; Manef, A. Chemical composition of some Tunisian Eucalyptus essential oils as obtained by hydrodistillation using clevenger type apparatus. Biosci. Biotechnol. Res. Asia 2010, 7, 647–656. [Google Scholar]
- Danna, C.; Cornara, L.; Smeriglio, A.; Trombetta, D.; Amato, G.; Aicardi, P.; De Martino, L.; De Feo, V.; Caputo, L. Eucalyptus gunnii and Eucalyptus pulverulenta ‘Baby Blue’ Essential Oils as Potential Natural Herbicides. Molecules 2021, 26, 6749. [Google Scholar] [CrossRef]
- Elaissi, A.; Marzouki, H.; Medini, H.; Larbi Khouja, M.; Farhat, F.; Lynene, F.; Harzallah-Skhiri, F.; Chemli, R. Variation in Volatile Leaf Oils of 13 Eucalyptus Species Harvested from Souinet Arboreta (Tunisia). Chem. Biodivers. 2010, 7, 909–921. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Hardel, D.K.; Sahoo, L. A review on phytochemical and pharmacological of Eucalyptus globules: A multipurpose tree. Int. J. Res. Ayurveda Pharm. 2011, 2, 1527–1530. [Google Scholar]
- Boland, D.J.; Brooker, M.I.H.; Turnbull, J.W. Eucalyptus Seed; CSIRO Publishing: Collingwood, Australia, 1980. [Google Scholar]
- Small, B.E.J. The Australian eucalyptus oil industry—An overview. J. Aust. For. 2013, 44, 170–177. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef] [Green Version]
- Ben Marzoug, H.N.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Larbi Khouja, M.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef]
- Valeriano, C.; Oliveira, T.L.C.; Carvalho, S.M.; Cardoso, M.G.; Alves, E.; Piccoli, R.H. The sanitizing action of essential oil-based solutions against Salmonella enterica serotype Enteritidis S64 biofilm formation on AISI 304 stainless steel. Food Control 2012, 25, 673–677. [Google Scholar] [CrossRef]
- Ameur, E.; Sarra, M.; Yosra, D.; Mariem, K.; Nabil, A.; Lynen, F.; Larbi, K.M. Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complement. Med. Ther. 2021, 21, 209. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Quert, R.; Garcia, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) K. D. Hill & L. A. S. Johnson, grown in Cuba. Flavour Fragr. J. 2002, 17, 1–4. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Salem, N.A.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; et al. Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.B.; An, M.; Wu, H.; Stanton, R.; Lemerle, D. Eucalyptus essential oils: Chemistry and bioactivity. Allelopathy J. 2010, 25, 313–330. [Google Scholar]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Ghalem, B.R.; Mohamed, B. Antibacterial activity of leaf essential oils of Eucalyptus globulus and Eucalyptus camaldulensis. Afr. J. Pharm. Pharmacol. 2008, 2, 211–215. [Google Scholar]
- Khammassi, M.; Polito, F.; Amri, I.; Khedhri, S.; Hamrouni, L.; Nazzaro, F.; Fratianni, F.; De Feo, V. Chemical Composition and Phytotoxic, Antibacterial and Antibiofilm Activity of the Essential Oils of Eucalyptus occidentalis, E. striaticalyx and E. stricklandii. Molecules 2022, 27, 5820. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Setia, N.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Phytotoxicity of volatile oil from Eucalyptus citriodora against some weedy species. J. Environ. Biol. 2007, 28, 63–66. [Google Scholar]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of essential oils as bioherbicides. J. Essent. Oil Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Holeman, M.; Rombourg, M.; Fechtal, M.; Gorrichon, J.P.; Lassaigne, G. Eucalyptus astringens Maiden, Eucalyptus blakelyi Maiden and Eucalyptus bosistoana F. Muell.: The same chemotype. Plantes Med. Phytother. 1987, 21, 311–316. [Google Scholar]
- Bouzabata, A.; Bighelli, A.; Abed, L.; Casanova, J.; Tomi, F. Composition and chemical variability of Eucalyptus bosistoana essential oil from Algerian Sahara. Nat. Prod. Commun. 2014, 9, 701–702. [Google Scholar] [CrossRef] [Green Version]
- Zrira, S.S.; Benjilali, B.B. Seasonal Changes in the Volatile Oil and Cineole Contents of Five Eucalyptus Species Growing in Morocco. J. Essent. Oil Res. 1996, 8, 19–24. [Google Scholar] [CrossRef]
- Sadeghi, H.; Reza, N.A.; Yoosefi, S. Essential leaf oil and nuclear ribosomal DNA sequence variation in six Eucalyptus populations. J. Essent. Oil Res. 2014, 26, 377–384. [Google Scholar] [CrossRef]
- Eid, H.H.; Ibrahim, T.A.; Sleem, A.A. Phytochemical and biological study of essential oils of four Eucalyptus species grown in Egypt. Egypt. J. Biomed. Sci. 2007, 24, 242–259. [Google Scholar]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi Khouja, M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial activity and chemical composition of 20 Eucalyptus species’ essential oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Elaissi, A.; Medini, H.; Rouis, Z.; Larbi Khouja, M.; Chemli, R.; Harzallah-Skhiri, F. Correlation between the chemotaxonomic classifications of the essential oils of 48 Eucalyptus species harvested from Tunisia and their phylogenetic classification. J. Exp. Biol. Agric. Sci. 2014, 2, 98–112. [Google Scholar]
- Elaissi, A.; Moumni, S.; Roeleveld, K.; Larbi Khouja, M. Chemical Characterization of Five Tunisian Eucalyptus Essential Oils Species. Chem. Biodivers. 2020, 17, e1900378. [Google Scholar] [CrossRef]
- Elaissi, A.; Cheraief, I.; Bannour, F.; Farhat, F.; Ben Salah, M.; Chemli, R.; Larbi Khouja, M. Contribution to the qualitative and quantitative study of seven Eucalyptus species’ essential oil harvested in Hajeb’s Layoun Arboreta (Tunisia). J. Essent. Oil Bear. Plants 2007, 10, 15–25. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Role of Monoterpenes in Eucalyptus Communities. Curr. Bioact. Compd. 2012, 8, 101–107. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Kohli, R.K. Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Z. Naturforsch. 2006, 61, 52–56. [Google Scholar] [CrossRef]
- Azizi, M.; Fuji, Y. Allelopathic effect of some medicinal plant substances on seed germination of Amaranthus retroflexus and Portulaca oleracea. Acta Horticult. 2006, 699, 61–68. [Google Scholar] [CrossRef]
- Kohli, R.K.; Singh, D. Allelopathic impact of volatile components from Eucalyptus on crop plants. Biol. Plant. 1991, 33, 475–483. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Ramezani, H.; Kohli, R.K. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 2002, 141, 111–116. [Google Scholar] [CrossRef]
- Kordali, S.; Cakirb, A.; Sutaya, S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z. Naturforsch. 2007, 62, 207–214. [Google Scholar] [CrossRef] [Green Version]
- De Martino, L.; Mancini, E.; De Almeida, L.F.R.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; Marandino, A.; De Almeida, L.F.; De Feo, V. Chemistry and antigerminative activity ofessential oils and monoterpenoids from Mediterranean plants. Curr. Bioact. Compd. 2012, 8, 13–49. [Google Scholar]
- Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef]
- He, Y.; Sang, S.; Tang, H.; Ou, C. In vitro mechanism of antibacterial activity of eucalyptus essential oil against specific spoilage organisms in aquatic products. J. Food Process. Preserv. 2022, 46, e16349. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538. [Google Scholar] [CrossRef]
- Robenshtok, E.; Paul, M.; Leibovici, L.; Fraser, A.; Pitlik, S.; Ostfeld, I.; Samra, Z.; Perez, S.; Lev, B.; Weinberger, M. The significance of Acinetobacter baumannii bacteremia compared with Klebsiella pneumoniae bacteremia: Risk factors and outcomes. J. Hosp. Infect. 2006, 64, 282–287. [Google Scholar] [CrossRef]
- Talbot, G.H.; Bradley, J.; Edwards, J.E., Jr.; Gilbert, D.; Scheld, M.; Bartlett, J.G. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 42, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Mead, P.S.; Griffin, P.M. Escherichia coli O157:H7. Lancet 1998, 352, 1207–1212. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Doyle, M.P. Foodborne disease significance of Escherichia coli O157:H7 and other enterohemorrhagic E. coli. Food Technol. 1997, 51, 69–76. [Google Scholar]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Abdollahzadeh, E.; Rezaei, M.; Hosseini, H. Antibacterial activity of plant essential oils and extracts: The role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control 2014, 35, 177–183. [Google Scholar] [CrossRef]
- Fridkin, S.K.; Hageman, J.C.; Morrison, M.; Sanza, L.T.; Como-Sabetti, K.; Jernigan, J.A.; Harriman, K.; Harrison, L.H.; Lynfield, R.; Farley, M.M. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 2005, 352, 1436–1444. [Google Scholar] [CrossRef]
- Prévost, G. Toxins in Staphylococcus aureus pathogenesis. In Microbial Toxins: Molecular and Cellular Biology; Proft, Ed.; Horizon Bioscience: Norfolk, UK, 2005; pp. 243–284. [Google Scholar]
- Lentino, J.R. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin. Infect. Dis. 2003, 36, 1157–1161. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Chaleshtori, F.S.; Saholi, M.; Chaleshtori, R.S. Chemical Composition, Antioxidant and Antibacterial Activity of Bunium persicum, Eucalyptus globulus, and Rose Water on Multidrug-Resistant Listeria Species. J. Evid.-Based Integr. Med. 2018, 23, 2515690X17751314. [Google Scholar] [CrossRef] [Green Version]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In Vitro Activity of Essential Oils against Planktonic and Biofilm Cells of Extended-Spectrum β-Lactamase (ESBL)/Carbapenamase-Producing Gram-Negative Bacteria Involved in Human Nosocomial Infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef]
- Caputo, L.; Smeriglio, A.; Trombetta, D.; Cornara, L.; Trevena, G.; Valussi, M.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemical Composition and Biological Activities of the Essential Oils of Leptospermum petersonii and Eucalyptus gunnii. Front. Microbiol. 2020, 11, 409. [Google Scholar] [CrossRef] [Green Version]
- Lagha, R.; Ben Abdallah, F.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [Green Version]
- Al-Qaysi, A.-M.K.; Al-Ouqaili, M.T.S.; Al-Meani, S.A.L. Ciprofloxacin- and gentamicin-mediated inhibition of Pseudomonas aeruginosa biofilms is enhanced when combined the volatile oil from Eucalyptus camaldulensis. Syst. Rev. Pharm. 2020, 11, 98–105. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT-Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Springer: New York, NY, USA, 1994. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zaval, J.F.; Coppola, R.; Nazzaro, F. Fatty acid composition and in vitro anti-inflammatory activity of five cold-pressed Prunus seed oils, and their anti-biofilm effect against pathogenic bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef]
| KI a | KI b | Compound | Eb | Em | Eo | Ep | Es | Etr | Identification c |
|---|---|---|---|---|---|---|---|---|---|
| 770 | n-Octane | t | - | - | - | - | - | 1,2 | |
| 864 | 1012 | α-Pinene | 1.1 | 8.2 | 1.5 | 1.5 | 10.9 | 14.8 | 1,2,3 |
| 876 | 1092 | Camphene | t | 0.2 | 0.1 | - | 0.1 | 0.1 | 1,2,3 |
| 883 | 1115 | Thuja-2,4(10)-diene | t | - | - | - | - | - | 1,2 |
| 902 | 1110 | β-Pinene | - | - | 2.5 | 3.4 | 0.5 | 0.4 | 1,2,3 |
| 903 | 1205 | Limonene | t | - | - | - | - | - | 1,2,3 |
| 922 | 1145 | Myrcene | - | - | - | - | - | 0.1 | 1,2,3 |
| 929 | 1177 | α-Phellandrene | - | - | 0.2 | - | 0.1 | 0.1 | 1,2,3 |
| 941 | 1170 | α-Terpinene | - | - | 0.3 | - | - | - | 1,2,3 |
| 952 | 1250 | a-Cymene | - | - | 25.4 | 1.6 | 2.1 | - | 1,2,3 |
| 957 | 1210 | Eucalyptol | 75.2 | 78.1 | 6.8 | 44.9 | 70.8 | 48.4 | 1,2,3 |
| 983 | 1221 | γ-Terpinene | - | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 1,2,3 |
| 996 | 1115 | cis-Sabinene hydrate | - | - | 0.4 | - | - | - | 1,2,3 |
| 997 | cis -Linalool oxide | - | - | - | 0.1 | - | - | 1,2,3 | |
| 1008 | 1291 | Terpinolene | - | - | 0.3 | 0.5 | - | - | 1,2,3 |
| 1010 | 1250 | p-Cymenene | - | - | 0.6 | - | - | - | 1,2 |
| 1011 | 1384 | α-pinene oxide | t | - | - | - | - | - | 1,2 |
| 1023 | 1506 | Linalool | - | - | 1.1 | 1.3 | - | - | 1,2,3 |
| 1029 | Pentanoic acid, pentyl ester | - | 0.3 | - | - | 0.7 | 0.3 | 1,2 | |
| 1031 | exo-Fenchol | 0.2 | 0.6 | - | - | - | 0.5 | 1,2 | |
| 1032 | Isopinocampheol | - | - | - | - | 0.3 | - | 1,2 | |
| 1040 | 1474 | trans-Sabinene hydrate | - | - | 0.3 | - | - | - | 1,2 |
| 1042 | 1485 | α-Campholenal | - | - | 1.3 | - | - | 0.1 | 1,2 |
| 1043 | 3-Cyclopentene-1-acetaldehyd | - | 0.1 | - | - | - | - | 1,2 | |
| 1044 | 6-Camphenol | - | - | - | 0.6 | 0.1 | 0.2 | 1,2 | |
| 1051 | Nopinone | - | - | 0.5 | - | - | - | 1,2 | |
| 1052 | 1382 | allo-Ocimene | 0.3 | 0.1 | - | - | 0.1 | 0.1 | 1,2,3 |
| 1055 | 1720 | trans-Sabinol | - | - | 3.6 | - | - | - | 1,2 |
| 1056 | 1753 | Cumin aldehyde | - | - | 2.4 | 1.2 | - | - | 1,2 |
| 1058 | 1664 | trans-Pinocarveol | 6.8 | 3.8 | - | 10.8 | 4,3 | 10.8 | 1,2 |
| 1062 | cis-Pinene hydrate | - | 0.1 | - | - | - | - | 1,2 | |
| 1063 | 1663 | cis-Verbenol | t | - | - | - | - | - | 1,2 |
| 1065 | cis-β-terpineol | - | - | 0.5 | - | - | 0.1 | 1,2 | |
| 1069 | 1643 | Sabina ketone | - | - | 7.7 | 1.1 | - | - | 1,2 |
| 1075 | trans-Pinocamphone | - | - | - | - | - | 0.1 | 1,2 | |
| 1076 | 1468 | trans-Limonene oxide | t | - | - | - | - | - | 1,2 |
| 1078 | 1580 | Pinocarvone | 1.3 | 0.8 | 1.5 | 2.8 | 1.1 | 2.9 | 1,2,3 |
| 1082 | 1715 | Borneol | 0.3 | 1.2 | 1.3 | 2.2 | 0.2 | 0.6 | 1,2,3 |
| 1084 | neo-Iso-isopulegol | - | 0.2 | - | - | - | - | 1,2 | |
| KI a | KI b | Compound | Eb | Em | Eo | Ep | Es | Etr | Identification c |
| 1085 | 1832 | trans-Carveol | 0.2 | - | - | - | - | - | 1,2 |
| 1086 | Menthol | - | - | - | - | 0.1 | - | 1,2,3 | |
| 1087 | cis-Pinocamphone | - | - | - | - | - | 0.2 | 1,2 | |
| 1092 | cis-Pulegol | t | - | - | - | - | - | 1,2 | |
| 1093 | 1590 | Terpinen-4-ol | - | 0.4 | 4 | 1.2 | 0.4 | 0.4 | 1,2,3 |
| 1097 | 1665 | neo-Verbanol | - | - | 7.9 | 1.8 | - | - | 1,2 |
| 1099 | 1678 | trans-p-Mentha-2,8-dien-1-ol | 0.9 | - | - | - | - | - | 1,2 |
| 1102 | 1661 | α-Terpineol | - | 3.3 | 6.7 | - | 0.8 | 0.8 | 1,2,3 |
| 1105 | 1828 | p-Cymen-8-ol | - | - | 1.7 | - | - | - | 1,2 |
| 1106 | cis-Pinocarveol | - | - | - | - | 0.3 | 0.1 | 1,2 | |
| 1107 | 1678 | cis-p-Mentha-2,8-dien-1-ol | 0.1 | - | - | - | - | - | 1,2 |
| 1108 | Myrtenal | - | - | - | - | - | 0.5 | 1,2 | |
| 1109 | 1661 | β-terpineol | - | - | - | 3.4 | - | - | 1,2,3 |
| 1110 | Dihydro carveol | - | 0.2 | - | 1.2 | - | - | 1,2 | |
| 1111 | 1720 | trans-Sabinol | t | - | - | - | - | - | 1,2 |
| 1115 | 1791 | Myrtenol | - | - | - | 2 | 0.5 | - | 1,2 |
| 1122 | trans-Dihydro carvone | - | - | - | - | - | 0.2 | 1,2 | |
| 1123 | 1726 | Verbenone | - | - | - | 1.5 | - | - | 1,2 |
| 1134 | 1683 | trans-Verbenol | 1.1 | - | - | - | - | - | 1,2 |
| 1142 | 1878 | trans-Carveol | - | - | 0.5 | - | 0.4 | 0.2 | 1,2 |
| 1150 | 1581 | Thymol, methyl ether | - | - | 7.4 | - | - | - | 1,2 |
| 1167 | 1717 | Citronellol | - | - | - | - | 0.2 | - | 1,2,3 |
| 1181 | 1720 | p-Menth-1-en-7-al | - | - | 4.6 | - | - | - | 1,2 |
| 1193 | 1491 | Camphor | t | - | - | - | - | - | 1,2,3 |
| 1198 | α-Terpinen-7-al | - | - | - | 0.2 | - | - | 1,2 | |
| 1211 | 1868 | Carvacrol acetate | - | - | - | 0.5 | - | - | 1,2 |
| 1230 | 2172 | Thymol | - | - | 2.1 | - | 0.7 | - | 1,2,3 |
| 1247 | exo-2-Hydroxycineole acetate | - | - | - | - | 0.2 | 1,2 | ||
| 1248 | Car-3-en-2-one | 0.1 | - | - | - | - | - | 1,2 | |
| 1248 | γ-Terpinen-7-al | - | - | - | - | 0.2 | - | 1,2 | |
| 1250 | 2219 | Carvacrol | - | - | - | - | 0.3 | - | 1,2 |
| 1253 | trans-Sabinene hydrate acetate | t | - | - | - | - | - | 1,2 | |
| 1327 | 1631 | Aromadendrene | - | - | - | - | 0.1 | 0.5 | 1,2,3 |
| 1332 | Presilphiperfol-7-ene | 0.8 | - | - | - | - | - | 1,2 | |
| 1337 | Silphinene | t | - | - | - | - | - | 1,2 | |
| 1349 | Longicyclene | 0.2 | - | - | - | - | - | 1,2 | |
| 1361 | α-Ylangene | t | - | - | - | - | - | 1,2 | |
| 1374 | 1660 | allo-Aromadendrene | - | - | - | - | - | 0.1 | 1,2,3 |
| 1384 | 1725 | β-Selinene | - | - | - | - | - | 0.1 | 1,2 |
| 1386 | 1548 | β-Cubebene | 0.1 | - | - | - | - | - | 1,2,3 |
| 1408 | Viridiflorene | - | - | 0.2 | 0.1 | - | 0.6 | 1,2 | |
| 1433 | 1574 | Longifolene | t | - | - | 0.1 | - | - | 1,2 |
| KI a | KI b | Compound | Eb | Em | Eo | Ep | Es | Etr | Identification c |
| 1434 | 1957 | epi-Cubebol | - | - | - | 0.2 | - | - | 1,2 |
| 1441 | γ-Patchoulene | - | - | - | 0.2 | - | - | 1,2 | |
| 1444 | 2096 | Epiglobulol | 0.5 | - | - | - | - | - | 1,2 |
| 1448 | α-Guaiene | 0.4 | - | - | - | - | - | 1,2 | |
| 1459 | 2127 | Spathulenol | - | - | 2.4 | 4.2 | 0.3 | - | 1,2 |
| 1460 | α-Himalachene | 1.6 | - | - | - | - | - | 1,2 | |
| 1462 | cis-Eudesma-6,11-diene | 0.2 | - | - | - | - | - | 1,2 | |
| 1464 | 2110 | Viridiflorol | - | - | 0.8 | 5.2 | - | 0.2 | 1,2 |
| 1465 | (-)-Globulol | - | - | - | - | 0.5 | - | 1,2,3 | |
| 1466 | Patchoulene | - | - | - | - | - | 2.9 | 1,2 | |
| 1467 | 1722 | β-Selinene | 3.5 | - | - | - | - | - | 1,2 |
| 1472 | trans-β-Guaiene | - | - | - | 0.1 | - | 0.4 | 1,2 | |
| 1473 | 1748 | cis-β-guaiene | 0.8 | - | - | - | - | - | 1,2 |
| 1474 | 1752 | γ-Cadinene | - | - | - | - | - | 0.1 | 1,2 |
| 1475 | 10-epi-Cubebol | 0.3 | - | - | - | - | - | 1,2 | |
| 1482 | Modhephen-8-β-ol | - | - | - | - | - | 0.2 | 1,2 | |
| 1483 | allo-Cedrol | 0.3 | - | - | - | - | - | 1,2 | |
| 1495 | Rosifoliol | 0.2 | - | - | - | - | 0.2 | 1,2 | |
| 1497 | (E)-γ-Bisabolene | 0.1 | - | - | - | - | - | 1,2 | |
| 1501 | 2080 | Cubenol | - | 0.7 | 0.7 | - | - | 1,2 | |
| 1505 | 2178 | γ-Eudesmol | - | - | - | - | 2 | 1,2 | |
| 1515 | β-Eudesmol | - | 0.3 | 0.9 | 0.8 | 4.3 | 1,2 | ||
| 1523 | 2247 | α-Eudesmol | - | - | 1.3 | - | 3.3 | 1,2 | |
| Total | 96.6 | 97.8 | 97.9 | 97.0 | 97.0 | 97.2 | |||
| Monoterpene hydrocarbons | 1.4 | 8.7 | 30.3 | 6.7 | 13.9 | 15.7 | |||
| Oxygentated monoterpenes | 86.2 | 89.0 | 62.7 | 77.3 | 81.4 | 66.6 | |||
| Sesquiterpene hydrocarbons | 7.7 | 0 | 0.2 | 0.5 | 0.1 | 4.7 | |||
| Oxygentated sesquiterpenes | 1.3 | 0 | 4.2 | 12.5 | 1.6 | 10.2 | |||
| Others | 0.0 | 0.1 | 0.5 | 0.0 | 00 | 0.0 |
| R. sativus Germination | ||||||
|---|---|---|---|---|---|---|
| Dose (µg/mL) | E. bosistoana | E. melliodora | E. odorata | E. paniculata | E. salmonopholia | E. transcontinentalis |
| Control | 9.3 ± 1.2 | 9.7 ± 0.6 | 7.7 ± 0.6 | 8.0 ± 1.0 | 6.3 ± 1.5 | 8.0 ± 1.7 |
| 125 | 6.7 ± 1.2 ** | 9.3 ± 1.2 | 4.0 ± 1.0 **** | 0.7 ± 0.6 **** | 5.3 ± 2.5 | 6.3 ± 1.5 |
| 250 | 4.7 ± 0.6 **** | 9.7 ± 0.6 | 1.3 ± 0.6 **** | 0.7 ± 0.6 **** | 5.7 ± 2.1 | 3.7 ± 0.6 **** |
| 500 | 1.3 ± 0.6 **** | 9.7 ± 0.6 | 0.3 ± 0.6 **** | 0.0 ± 0.0 **** | 1.7 ± 2.1 **** | 1.3 ± 1.2 **** |
| 1000 | 0.0 ± 0.0 **** | 6.3 ± 1.5 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.3 ± 0.6 **** | 0.0 ± 0.0 **** |
| R. sativus Radical elongation | ||||||
| Control | 3.0 ± 0.1 | 6.3 ± 0.2 | 2.7 ± 0.3 | 3.2 ± 0.3 | 1.4 ± 0.1 | 4.0 ± 0.2 |
| 125 | 2.1 ± 0.3 **** | 6.1 ± 0.4 | 1.7 ± 0.2 **** | 1.0 ± 1.0 **** | 1.0 ± 0.1 | 2.3 ± 0.6 * |
| 250 | 1.6 ± 0.3 **** | 4.1 ± 0.3 **** | 0.6 ± 0.1 **** | 0.4 ± 0.4 **** | 1.1 ± 0.3 | 1.9 ± 0.5 ** |
| 500 | 0.6 ± 0.3 **** | 3.1 ± 0.5 **** | 0.1 ± 0.1 **** | 0.0 ± 0.0 **** | 0.6 ± 0.5 | 2.2 ± 2.0 * |
| 1000 | 0.0 ± 0.0 **** | 2.0 ± 0.1 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.1 ± 0.2 * | 0.0 ± 0.0 **** |
| S. arvensis Germination | ||||||
| Dose (µg/mL) | E. bosistoana | E. melliodora | E. odorata | E. paniculata | E. salmonopholia | E. transcontinentalis |
| Control | 10.0 ± 0.0 | 10.0 ± 0.0 | 9.7 ± 0.6 | 9.7 ± 0.6 | 10.0 ± 0.0 | 10.0 ± 0.0 |
| 125 | 9.3 ± 0.6 | 9.0 ± 1.0 | 0.0 ± 0.0 **** | 1.7 ± 0.6 **** | 9.7 ± 0.6 | 9.7 ± 0.6 |
| 250 | 4.0 ± 1.0 **** | 5.3 ± 1.2 **** | 0.0 ± 0.0 **** | 0.3 ± 0.6 **** | 6.7 ± 1.5 * | 5.3 ± 1.5 **** |
| 500 | 1.0 ± 1.0 **** | 1.3 ± 0.6 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.7 ± 0.6 **** | 1.0 ± 1.7 **** |
| 1000 | 0.0 ± 0.0 **** | 0.3 ± 0.6 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** |
| S. arvensis Radical elongation | ||||||
| Control | 3.8 ± 0.1 | 6.5 ± 0.6 | 6.7 ± 0.2 | 3.9 ± 0.1 | 3.8 ± 0.8 | 3.8 ±0.8 |
| 125 | 1.3 ± 0.1 **** | 2.4 ± 0.7 **** | 0.0 ± 0.0 **** | 0.8 ± 0.1 **** | 2.5 ± 0.0 * | 1.9 ± 0.7 ** |
| 250 | 0.6 ± 0.2 **** | 1.2 ± 0.3 **** | 0.0 ± 0.0 **** | 0.1 ± 0.2 **** | 1.4 ± 0.4 **** | 0.5 ± 0.2 **** |
| 500 | 0.7 ± 0.6 **** | 0.7 ± 0.4 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.6 ± 0.7 **** | 0.4 ± 0.6 **** |
| 1000 | 0.0 ± 0.0 **** | 0.1 ± 0.2 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** |
| L. multiflorum Germination | ||||||
| E. bosistoana | E. melliodora | E. odorata | E. paniculata | E. salmonopholia | E. transcontinentalis | |
| Control | 9.3 ± 0.6 | 8.3 ± 0.6 | 8.7 ± 1.2 | 9.3 ± 0.6 | 9.7 ± 0.6 | 9.7 ± 0.6 |
| 125 | 9.0 ± 1.0 | 8.0 ± 1.0 | 7.3 ± 1.2 | 9.0 ± 1.0 | 9.7 ± 0.6 | 8.7 ± 0.6 |
| 250 | 8.7 ± 1.2 | 8.0 ± 1.0 | 6.3 ± 0.6 **** | 8.7 ± 0.6 | 8.3 ± 0.6 | 8.0 ± 1.7 |
| 500 | 8.3 ± 0.6 | 7.3 ± 1.2 | 3.7 ± 0.6 **** | 5.3 ± 0.6 **** | 9.3 ± 0.6 | 8.0 ± 1.0 |
| 1000 | 4.7 ± 0.6 **** | 6.3 ± 0.6 | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 7.7 ± 0.6 | 1.0 ± 1.0 **** |
| L. multiflorum Radical elongation | ||||||
| Control | 4.7 ± 0.2 | 6.5 ± 0.2 | 5.4 ± 0.6 | 4.9 ± 0.2 | 5.1 ± 0.6 | 5.5 ± 0.3 |
| 125 | 3.2 ± 0.1 **** | 3.8 ± 0.3 **** | 1.6 ± 0.4 **** | 2.7 ± 0.3 **** | 5.5 ± 0.5 | 2.7 ± 0.2 **** |
| 250 | 2.0 ± 0.2 **** | 2.7 ± 0.2 **** | 0.7 ± 0.3 **** | 1.2 ± 0.2 **** | 4.2 ± 0.7 | 1.6 ± 0.3 **** |
| 500 | 0.9 ± 0.1 **** | 1.3 ± 0.3 **** | 0.3 ± 0.1 **** | 0.3 ± 0.1 **** | 3.5 ± 0.5 ** | 0.4 ± 0.2 **** |
| 1000 | 0.5 ± 0.2 **** | 0.5 ± 0.3 **** | 0.0 ± 0.0 **** | 0.0 ± 0.0 **** | 0.8 ± 0.3 **** | 0.1 ± 0.1 **** |
| MIC (µL/mL) | |||||
|---|---|---|---|---|---|
| A. baumannii | E. coli | L. monocytogenes | P. aeruginosa | S. aureus | |
| E. bosistoana | 27 ± 2 | 26 ± 2 * | 28 ± 2 | 28 ± 1 | 24 ± 2 |
| E. melliodora | 30 ± 1 * | 24 ± 1 | 30 ± 1 * | 28 ±1 | 28 ± 1 |
| E. odorata | 28 ± 1 | 26 ± 1 * | 30 ± 1 * | 27 ± 1 | 27 ± 1 |
| E. paniculata | 26 ± 2 | 26 ± 1 * | 28 ± 1 | 30 ± 2 * | 28 ± 1 |
| E. salmonopholia | 30 ± 1 * | 28 ± 1 **** | 30 ± 1 * | 30 ± 1 * | 32 ± 1 **** |
| E. transcontinentalis | 28 ± 1 | 26 ± 1 * | 26 (±1) | 28 ± 1 | 28 ± 1 |
| Tetracycicline | 27 ± 1 | 23 ± 1 | 27 (±2) | 26 ± 2 | 26 ± 1 |
| Time 0 | A. baumannii | E. coli | L. monocytogenes | P. aeruginosa | S. aureus |
|---|---|---|---|---|---|
| E. bosistoana 10 µL/mL | 59.40 ± 0.60 **** | 68.52 ± 0.30 **** | 57.71 ± 0.22 **** | 63.26 ± 0.15 **** | 65.76 ± 0.09 **** |
| E. bosistoana 20 µL/mL | 76.69 ± 0.28 **** | 78.83 ± 0.22 **** | 58.77 ± 0.18 **** | 74.77 ± 0.14 **** | 69.39 ± 0.15 **** |
| E. melliodora 10 µL/mL | 8.93 ± 0.78 **** | 13.39 ± 0.64 **** | 59.39 ± 0.22 **** | 51.21 ± 0.29 **** | 73.87 ± 0.08 **** |
| E. melliodora 20 µL/mL | 25.44 ± 0.38 **** | 30.35 ± 0.20 **** | 73.31 ± 0.23 **** | 60.28 ± 0.16 **** | 76.40 ± 0.09 **** |
| E. odorata 10 µL/mL | 88.56 ± 0.14 **** | 86.26 ± 0.19 **** | 78.83 ± 0.19 **** | 75.39 ± 0.18 **** | 82.48 ± 0.07 **** |
| E. odorata 20 µL/mL | 92.59 ± 0.27 **** | 88.09 ± 0.20 **** | 79.57 ± 0.14 **** | 77.72 ± 0.15 **** | 85.77 ± 0.05 **** |
| E. paniculata 10 µL/mL | 89.63 ± 2.51 **** | 87.63 ± 2.45 **** | 89.71 ± 1.03 **** | 90.93 ± 0.58 **** | 85.35 ± 0.76 **** |
| E. paniculata 20 µL/mL | 93.01 ± 0.62 **** | 90.89 ± 0.32 **** | 91.65 ± 1.15 **** | 93.50 ± 0.97 **** | 87.02 ± 0.49 **** |
| E. salmonopholia 10 µL/mL | 59.10 ± 0.15 **** | 79.22 ± 0.08 **** | 67.97 ± 0.20 **** | 72.51 ± 0.11 **** | 48.68 ± 2.06 **** |
| E. salmonopholia 20 µL/mL | 66.06 ± 0.09 **** | 85.00 ± 0.09 **** | 69.88 ± 0.05 **** | 81.41 ± 0.13 **** | 58.45 ± 0.16 **** |
| E. transcontinentalis 10 µL/mL | 91.50 ± 0.11 **** | 79.69 ± 0.16 **** | 89.25 ± 0.06 **** | 83.74 ± 0.04 **** | 87.72 ± 0.05 **** |
| E. transcontinentalis 20 µL/mL | 93.99 ± 0.10 **** | 91.02 ± 0.07 **** | 90.40 ± 0.12 **** | 83.89 ± 0.08 **** | 90.19 ± 0.18 **** |
| Time 24 h | |||||
| E. bosistoana 10 µL/mL | 39.70 ± 1.11 **** | 39.23 ± 0.83 **** | 17.69 ± 2.00 **** | 45.62 ± 1.37 **** | 78.63 ± 0.50 **** |
| E. bosistoana 20 µL/mL | 58.01 ± 1.14 **** | 61.40 ± 0.80 **** | 46.21 ± 1.63 **** | 60.36 ± 0.75 **** | 79.84 ± 0.24 **** |
| E. melliodora 10 µL/mL | 28.50 ± 0.54 **** | 39.56 ± 0.37 **** | 35.73 ± 0.68 **** | 40.66 ± 0.75 **** | 75.06 ± 0.04 **** |
| E. melliodora 20 µL/mL | 36.37 ± 1.71 **** | 46.42 ± 0.91 **** | 37.44 ± 1.13 **** | 56.57 ± 0.70 **** | 80.01 ± 0.34 **** |
| E. odorata 10 µL/mL | 56.52 ± 0.71 **** | 33.71 ± 1.27 **** | 28.15 ± 0.88 **** | 49.83 ± 0.82 **** | 80.05 ± 0.20 **** |
| E. odorata 20 µL/mL | 59.10 ± 0.69 **** | 58.53 ± 0.94 **** | 33.73 ± 0.65 **** | 54.66 ± 1.65 **** | 80.63 ± 0.30 **** |
| E. paniculata 10 µL/mL | 83.02 ± 2.27 **** | 86.21 ± 2.54 **** | 89.47 ± 1.71 **** | 89.42 ± 0.61 **** | 78.27 ± 0.76 **** |
| E. paniculata 20 µL/mL | 85.95 ± 2.93 **** | 88.75 ± 1.84 **** | 93.63 ± 1.09 **** | 91.14 ± 0.62 **** | 85.38 ± 0.96 **** |
| E. salmonopholia 10 µL/mL | 30.79 ± 1.83 **** | 9.11 ± 0.91 **** | 54.59 ± 0.60 **** | 62.44 ± 0.69 **** | 49.62 ± 0.33 **** |
| E. salmonopholia 20 µL/mL | 51.03 ± 0.76 **** | 62.63 ± 1.44 **** | 63.61 ± 0.37 **** | 64.50 ± 0.64 **** | 53.76 ± 0.29 **** |
| E. transcontinentalis 10 µL/mL | 38.45 ± 0.27 **** | 27.15 ± 0.50 **** | 40.29 ± 0.36 **** | 60.57 ± 0.61 **** | 79.55 ± 0.31 **** |
| E. transcontinentalis 20 µL/mL | 47.47 ± 0.43 **** | 46.80 ± 0.91 **** | 41.69 ± 0.53 **** | 60.63 ± 0.63 **** | 81.29 ± 0.25 **** |
| Time 0 | A. baumannii | E. coli | L. monocytogenes | P. aeruginosa | S. aureus |
|---|---|---|---|---|---|
| E. bosistoana 10 µL/mL | 80.38 ± 1.48 **** | 48.90 ± 1.96 **** | 39.63 ± 2.20 **** | 72.41 ± 2.17 **** | 29.93 ± 0.92 **** |
| E. bosistoana 20 µL/mL | 83.99 ± 0.81 **** | 79.52 ± 1.83 **** | 73.01 ± 2.20 **** | 78.49 ± 0.77 **** | 80.94 ± 1.85 **** |
| E. melliodora 10 µL/mL | 66.85 ± 0.51 **** | 0.00 ± 0.00 ns | 77.69 ± 1.04 **** | 70.89 ± 2.91 **** | 78.41 ± 1.18 **** |
| E. melliodora 20 µL/mL | 70.01 ± 0.75 **** | 0.00 ± 0.00 ns | 79.78 ± 0.28 | 75.46 ± 1.29 **** | 80.71 ± 0.79 |
| E. odorata 10 µL/mL | 83.36 ± 0.67 **** | 75.29 ± 1.48 **** | 80.04 ± 1.28 **** | 79.72 ± 1.18 **** | 79.03 ± 2.67 **** |
| E. odorata 20 µL/mL | 85.12 ± 0.70 **** | 78.23 ± 3.98 **** | 83.22 ± 0.97 **** | 81.93 ± 0.37 **** | 81.77 ± 0.30 **** |
| E. paniculata 10 µL/mL | 50.43 ± 6.88 **** | 68.65 ± 0.47 **** | 76.13 ± 0.52 **** | 69.05 ± 1.26 **** | 81.24 ± 0.97 **** |
| E. paniculata 20 µL/mL | 63.85 ± 4.11 **** | 34.75 ± 0.89 **** | 84.69 ± 0.22 **** | 81.83 ± 1.42 **** | 86.75 ± 1.65 **** |
| E. salmonopholia 10 µL/mL | 0.00 ± 0.00 ns | 0.00 ± 0.00 ns | 64.05 ± 2.437 **** | 0.00 ± 0.00 ns | 48.08 ± 0.63 **** |
| E. salmonopholia 20 µL/mL | 46.81 ± 2.00 **** | 22.36 ± 1.88 **** | 73.40 ± 1.30 **** | 10.88 ± 2.09 **** | 58.49 ± 0.63 **** |
| E. transcontinentalis 10 µL/mL | 48.03 ± 3.60 **** | 48.58 ± 1.76 **** | 68.64 ± 1.84 **** | 76.88 ± 2.60 **** | 85.57 ± 1.35 **** |
| E. transcontinentalis 20 µL/mL | 86.62 ± 2.14 **** | 83.74 ± 3.00 **** | 88.49 ± 1.73 **** | 76.47 ± 3.08 **** | 90.61 ± 1.83 **** |
| Time 24 h | |||||
| E. bosistoana 10 µL/mL | 2.10 ± 1.75 ns | 33.53 ± 0.39 **** | 29.90 ± 1.09 **** | 0.00 ± 0.00 ns | 30.89 ± 1.52 **** |
| E. bosistoana 20 µL/mL | 35.07 ± 1.51 **** | 51.35 ± 0.92 **** | 54.04 ± 0.88 **** | 7.09 ± 0.44 **** | 33.23 ± 0.52 **** |
| E. melliodora 10 µL/mL | 0.00 ± 0.00 ns | 16.49 ± 0.80 **** | 0.00 ± 0.00 ns | 4.17 ± 0.38 ** | 8.67 ± 0.68 **** |
| E. melliodora 20 µL/mL | 0.00 ± 0.00 ns | 38.93 ± 0.86 **** | 0.00 ± 0.00 ns | 17.09 ± 1.19 **** | 18.98 ± 0.42 **** |
| E. odorata 10 µL/mL | 0.00 ± 0.00 ns | 20.60 ± 0.00 **** | 0.00 ± 0.00 ns | 0.00 ± 0.00 ns | 7.95 ± 1.09 **** |
| E. odorata 20 µL/mL | 0.00 ± 0.00 ns | 58.69 ± 0.00 **** | 0.00 ± 0.00 ns | 0.00 ± 0.00 ns | 28.29 ± 1.05 **** |
| E. paniculata 10 µL/mL | 50.43 ± 6.88 **** | 68.65 ± 0.47 **** | 76.13 ± 0.52 **** | 69.05 ± 1.26 **** | 81.24 ± 0.97 **** |
| E. paniculata 20 µL/mL | 63.85 ± 4.11 **** | 34.75 ± 0.89 **** | 84.69 ± 0.22 **** | 81.83 ± 1.42 **** | 86.75 ± 1.65 **** |
| E. salmonopholia 10 µL/mL | 37.30 ± 0.52 **** | 52.61 ± 0.45 **** | 54.76 ± 0.47 **** | 55.14 ± 0.80 **** | 25.44 ± 1.74 **** |
| E. salmonopholia 20 µL/mL | 39.33 ± 0.68 **** | 72.10 ± 0.51 **** | 56.57 ± 0.87 **** | 60.74 ± 0.91 **** | 58.72 ± 2.46 **** |
| E. transcontinentalis 10 µL/mL | 60.94 ± 0.93 **** | 67.46 ± 0.75 **** | 57.14 ± 0.44 **** | 66.75 ± 0.58 **** | 42.86 ± 2.96 **** |
| E. transcontinentalis 20 µL/mL | 65.08 ± 0.48 **** | 70.81 ± 0.41 **** | 60.60 ± 1.37 **** | 72.90 ± 0.81 **** | 55.87 ± 1.61 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouki, H.; Polito, F.; De Martino, L.; Mabrouk, Y.; Hamrouni, L.; Amri, I.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemistry and Bioactivities of Six Tunisian Eucalyptus Species. Pharmaceuticals 2022, 15, 1265. https://doi.org/10.3390/ph15101265
Kouki H, Polito F, De Martino L, Mabrouk Y, Hamrouni L, Amri I, Fratianni F, De Feo V, Nazzaro F. Chemistry and Bioactivities of Six Tunisian Eucalyptus Species. Pharmaceuticals. 2022; 15(10):1265. https://doi.org/10.3390/ph15101265
Chicago/Turabian StyleKouki, Habiba, Flavio Polito, Laura De Martino, Yassine Mabrouk, Lamia Hamrouni, Ismail Amri, Florinda Fratianni, Vincenzo De Feo, and Filomena Nazzaro. 2022. "Chemistry and Bioactivities of Six Tunisian Eucalyptus Species" Pharmaceuticals 15, no. 10: 1265. https://doi.org/10.3390/ph15101265