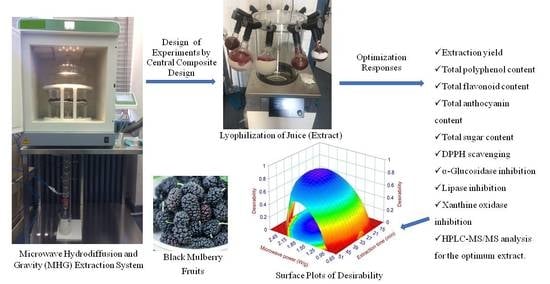
Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the Experiments (DoE) Analysis
2.2. MHG Optimization and Model Validation
2.3. Application of the HPLC-MS/MS Method to the Validated MHG Run
2.4. Comparison between MHG Validation Run Extract and Conventional Solvent Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.2.1. HPLC Analysis
3.2.2. Biological Studies
3.3. Extraction Procedures
3.3.1. Microwave Hydrodiffusion and Gravity (MHG) Extraction
3.3.2. Conventional Solvent Extraction
3.4. Design of the Experiments (DoE)
3.4.1. Central Composite Design
- (1)
- Extraction yield %, calculated as the weight of lyophilized extract per dry weight of fruit.
- (2)
- Total phenolic content (TPC), determined as reported in Section 3.6.
- (3)
- Total flavonoid content (TFC), determined as reported in Section 3.7.
- (4)
- Total anthocyanin content (TAC), determined as reported in Section 3.8.
- (5)
- Total sugar content (TSC), determined as reported in Section 3.9.
- (6)
- α-Glucosidase inhibition, determined as reported in Section 3.10.
- (7)
- Lipase inhibition, determined as reported in Section 3.11.
- (8)
- Xanthine oxidase inhibition, determined as reported in Section 3.12.1.
- (9)
- DPPH radical scavenging, determined as reported in Section 3.12.2.
3.4.2. Optimization and Validation
3.5. HPLC-ESI-MS/MS Analysis of the Validation MHG Run
3.6. Total Phenolic Content (TPC)
3.7. Total Flavonoid Content (TFC)
3.8. Total Anthocyanin Content (TAC)
3.9. Total Sugar Content (TSC)
3.10. α-Glucosidase Inhibition
3.11. Lipase Inhibition
3.12. Free Radical Scavenging Activity
3.12.1. Xanthine/Xanthine Oxidase Inhibition
3.12.2. DPPH Radical Scavenging
3.13. Moisture Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hojjatpanah, G.; Fazaeli, M.; Emam-Djomeh, Z. Effects of heating method and conditions on the quality attributes of black mulberry (Morus nigra) juice concentrate. Int. J. Food Sci. Technol. 2011, 46, 956–962. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of maturity on phenolics (phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiloglu, S.; Serali, O.; Unal, N.; Capanoglu, E. Antioxidant activity and polyphenol composition of black mulberry (Morus nigra L.) products. J. Berry Res. 2013, 3, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015, 186, 277–284. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Wang, R.-J.; Hu, M.-L. Antioxidant capacities of fruit extracts of five mulberry genotypes with different assays and principle components analysis. Int. J. Food Prop. 2011, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef]
- Song, N.; Yang, H.; Pang, W.; Qie, Z.; Lu, H.; Tan, L.; Li, H.; Sun, S.; Lian, F.; Qin, C. Mulberry extracts alleviate Aβ25–35-induced injury and change the gene expression profile in PC12 cells. Evid. Based Complement. Altern. Med. 2014, 2014, 150617. [Google Scholar] [CrossRef]
- Jiang, D.-Q.; Guo, Y.; Xu, D.-H.; Huang, Y.-S.; Yuan, K.; Lv, Z.-Q. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem. Toxicol. 2013, 59, 1–7. [Google Scholar] [CrossRef]
- Chan, K.-C.; Ho, H.-H.; Lin, M.-C.; Yen, C.-H.; Huang, C.-N.; Huang, H.-P.; Wang, C.-J. Mulberry water extracts inhibit atherosclerosis through suppression of the integrin-β3/focal adhesion kinase complex and downregulation of nuclear factor κB signaling in vivo and in vitro. J. Agric. Food Chem. 2014, 62, 9463–9471. [Google Scholar]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crop. Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Périno, S.; Pierson, J.T.; Ruiz, K.; Cravotto, G.; Chemat, F. Laboratory to pilot scale: Microwave extraction for polyphenols lettuce. Food Chem. 2016, 204, 108–114. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT Food Sci. Technol. 2008, 41, 652–659. [Google Scholar] [CrossRef]
- Vian, M.A.; Fernandez, X.; Visinoni, F.; Chemat, F. Microwave hydrodiffusion and gravity, a new technique for extraction of essential oils. J. Chromatogr. A 2008, 1190, 14–17. [Google Scholar] [CrossRef]
- Ravi, H.K.; Breil, C.; Vian, M.A.; Chemat, F.; Venskutonis, P.R. Biorefining of bilberry (Vaccinium myrtillus L.) pomace using microwave hydrodiffusion and gravity, ultrasound-assisted, and bead-milling extraction. ACS Sustain. Chem. Eng. 2018, 6, 4185–4193. [Google Scholar] [CrossRef]
- Mazzara, E.; Scortichini, S.; Fiorini, D.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Morgese, G.; Morshedloo, M.R.; Palmieri, G.F.; Cespi, M. A design of experiment (DoE) approach to model the yield and chemical composition of ajowan (Trachyspermum ammi L.) essential oil obtained by microwave-assisted extraction. Pharmaceuticals 2021, 14, 816. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Demir, S.; Haznedaroglu, M.Z.; Yesil-Celiktas, O. Optimization of microwave assisted extraction of Morus nigra L. fruits maximizing tyrosinase inhibitory activity with isolation of bioactive constituents. Food Chem. 2018, 248, 183–191. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.; Cantos, E.; Palma, M.; Barroso, C. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Xue, H.; Xu, H.; Wang, X.; Shen, L.; Liu, H.; Liu, C.; Qin, Q.; Zheng, X.; Li, Q. Effects of microwave power on extraction kinetic of anthocyanin from blueberry powder considering absorption of microwave energy. J. Food Qual. 2018, 2018, 9680184. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, Y.; Xu, W.; Zheng, X. Analysis of the anthocyanin degradation in blue honeysuckle berry under microwave assisted foam-mat drying. Foods 2020, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.M.; Angeloni, S.; Abouelenein, D.; Acquaticci, L.; Xiao, J.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of 36 polyphenols in blueberry, strawberry and their commercial products and determination of antioxidant activity. Food Chem. 2022, 367, 130743. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Martínez, J.J.; Hernández, F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC–MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Okatan, V. Phenolic compounds and phytochemicals in fruits of black mulberry (Morus nigra L.) genotypes from the Aegean region in Turkey. Folia Hortic. 2018, 30, 93. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Simonoff, J.S. Handbook of Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 5. [Google Scholar]
- Mustafa, A.M.; Maggi, F.; Öztürk, N.; Öztürk, Y.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. Chemical and biological analysis of the by-product obtained by processing Gentiana lutea L. and other herbs during production of bitter liqueurs. Ind. Crop. Prod. 2016, 80, 131–140. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Abd-Allah, R.H.; Borsetta, G.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. Effect of roasting, boiling, and frying processing on 29 polyphenolics and antioxidant activity in seeds and shells of sweet chestnut (Castanea sativa mill.). Plants 2021, 10, 2192. [Google Scholar] [CrossRef] [PubMed]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S. Effect of plasma activated water (PAW) on rocket leaves decontamination and nutritional value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar] [CrossRef]
- Avalos-Llano, K.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Effect of pulsed light treatments on quality and antioxidant properties of fresh-cut strawberries. Food Chem. 2018, 264, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.L.; Tong, L.; Wei, L.; De Yang, L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crop. Prod. 2015, 69, 137–142. [Google Scholar] [CrossRef]
- Kazeem, M.; Adamson, J.; Ogunwande, I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Taviano, M.F.; Miceli, N.; Acquaviva, R.; Malfa, G.A.; Ragusa, S.; Giordano, D.; Cásedas, G.; Les, F.; López, V. Cytotoxic, antioxidant, and enzyme inhibitory properties of the traditional medicinal plant Matthiola incana (L.) R. Br. Biology 2020, 9, 163. [Google Scholar] [CrossRef]
- Lopez, V.; Akerreta, S.; Casanova, E.; García-Mina, J.; Cavero, R.; Calvo, M. Screening of Spanish medicinal plants for antioxidant and antifungal activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Hypoglycemic, anti-glycation and antioxidant in vitro properties of two Vaccinium species from Macaronesia: A relation to their phenolic composition. J. Funct. Foods 2018, 40, 595–605. [Google Scholar] [CrossRef]





| Response | Best Model a | R2 | R2adj | R2pred | Mallow’s Cp | p-Value regr b | p-Value LOF b |
|---|---|---|---|---|---|---|---|
| EY% | Y = −2.83 + 0.36P + 0.519T − 1.487P2 − 0.006T2 | 0.972 | 0.953 | 0.872 | 4.66 | *** | * |
| TPC (mg GAE/100 g) | Y = −3393 + 4135P +127.9T − 1343 P2 − 2.61 T2 + 39.4 P*T | 0.936 | 0.873 | 0.623 | 6 | ** | ns |
| TFC (mg RE/100 g) | Y = 1352 + 99P − 16.4T + 10.76 P*T | 0.654 | 0.506 | 0.00 | 2.08 | * | ns |
| TAC (mg CGE/100 g) | Y = 361 + 1008P − 13.01T − 327.6 P2 | 0.934 | 0.902 | 0.814 | 5.01 | *** | * |
| TSC (mg GE/100 g) | Y = 33,210 − 4034P − 495T + 239 P*T | 0.652 | 0.477 | 0.00 | 2.27 | ns | ** |
| AGHi IC50 (µg/mL) | Y = 442 − 578P + 15.1T +230P2 − 8.9P*T | 0.726 | 0.360 | 0.00 | 6.52 | ns | ** |
| Li IC50 (µg/mL) | Y = 10,447 − 8735P + 2260P2 | 0.823 | 0.779 | 0.625 | 4.11 | ** | ns |
| XOi IC50 (µg/mL) | Y = 2045 − 1348P − 33.8T + 304.7P2 + 0.455T2 | 0.913 | 0.855 | 0.58 | 4.02 | ** | ** |
| DPPH IC50 (µg/mL) | Y = 481.6 − 63.6P − 1.79T | 0.598 | 0.498 | 0.273 | 1.37 | * | ns |
| Run. | MAE Conditions | Composite Desirability | Responses Optimized with Desirability | Desirability Function | 95% Interval of Confidence | 95% Interval of Prediction | |
|---|---|---|---|---|---|---|---|
| Power (W/g) | Time (min) | ||||||
| 12 | 1.86 | 31.07 | 0.83 | EY% | Maximize | 12.4–14.9 | 11.1–16.2 |
| TPC | Maximize | 2906–3901 | 2379–4428 | ||||
| TAC | Maximize | 622–806 | 525–903 | ||||
| Li | Minimize | 1476–2536 | 597–3415 | ||||
| XOi | Minimize | 0–98.4 | 0–240 | ||||
| Run. | Point Type a | Coded Variables b | Uncoded Variables | Absolute Values | |||
|---|---|---|---|---|---|---|---|
| ET (min) | MP (W/g) | ET (min) | MP (W/g) | ET (min) | MP (W) | ||
| 1 | F | −1 | −1 | 15 | 1 | 15 | 500 |
| 2 | F | −1 | 1 | 15 | 2.4 | 15 | 1200 |
| 3 | F | 1 | −1 | 45 | 1 | 45 | 500 |
| 4 | F | 1 | 1 | 45 | 2.4 | 45 | 1200 |
| 5 | A | 0 | −1.41 | 30 | 0.7 | 30 | 355 |
| 6 | A | 0 | 1.41 | 30 | 2.7 | 30 | 1345 |
| 7 | A | −1.41 | 0 | 8.8 | 1.7 | 8.8 | 850 |
| 8 | A | 1.41 | 0 | 51.2 | 1.7 | 51.2 | 850 |
| 9 | C | 0 | 0 | 30 | 1.7 | 30 | 850 |
| 10 | C | 0 | 0 | 30 | 1.7 | 30 | 850 |
| 11 | C | 0 | 0 | 30 | 1.7 | 30 | 850 |
| No. | Compound | Concentration (mg/kg ± RSD%) |
|---|---|---|
| Anthocyanins | ||
| 1 | Delphindin-3,5-diglucoside | nd |
| 2 | Delphindin-3-galactoside | nd |
| 3 | Cyanidin-3-glucoside | 6016.72 ± 3.1 |
| 4 | Petunidin-3-glucoside | nd |
| 5 | Pelargonidin-3-rutinoside | 68.52 ± 3.2 |
| 6 | Pelargonidin-3-glucoside | 108.81 ± 4.5 |
| 7 | Malvidin-3-galactoside | nd |
| Flavonols | ||
| 8 | Quercetin | 46.49 ± 1.7 |
| 9 | Rutin | 222.9 ± 1.2 |
| 10 | Isoquercitrin | 94.4 ± 2.1 |
| 11 | Quercitrin | nd |
| 12 | Hyperoside | 207.67 ± 0.6 |
| 13 | Isorhamnetin | nd |
| 14 | Myricetin | nd |
| 15 | Kaempferol | 1.35 ± 4.3 |
| 16 | Kaempferol-3-glucoside | 7.85 ± 3.0 |
| Flavan-3-ols | ||
| 17 | (+)-Catechin | 1.92 ± 0.9 |
| 18 | (-)- Epicatechin | nd |
| 19 | Procyanidin A2 | nd |
| 20 | Procyanidin B2 | nd |
| Dihydrochalcones | ||
| 21 | Phloridzin | 1.41 ± 2.0 |
| 22 | Phloretin | 1.12 ± 1.2 |
| Flavanones | ||
| 23 | Hesperidin | nd |
| 24 | Naringin | nd |
| Phenolic acids | ||
| 25 | Neochlorogenic acid | 57.5 ± 1.2 |
| 26 | Chlorogenic acid | 565.6 ± 1.7 |
| 27 | Gallic acid | 4.0 ± 0.8 |
| 28 | p-Hydroxybenzoic acid | 0.4 ± 1.1 |
| 29 | 3-Hydroxybenzoic acid | nd |
| 30 | Caffeic acid | 3.9 ± 2.7 |
| 31 | Vanillic acid | 8.0 ± 1.3 |
| 32 | Syringic acid | 2.2 ± 1.12 |
| 33 | p-Coumaric acid | 8.8 ± 2.6 |
| 34 | Ferulic acid | 1.6 ± 3.3 |
| 35 | 3,5-Dicaffeoylquinic acid | 5.0 ± 2.6 |
| 36 | Ellagic acid | 51.66 ± 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, A.M.; Mazzara, E.; Abouelenein, D.; Angeloni, S.; Nunez, S.; Sagratini, G.; López, V.; Cespi, M.; Vittori, S.; Caprioli, G.; et al. Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design. Pharmaceuticals 2022, 15, 99. https://doi.org/10.3390/ph15010099
Mustafa AM, Mazzara E, Abouelenein D, Angeloni S, Nunez S, Sagratini G, López V, Cespi M, Vittori S, Caprioli G, et al. Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design. Pharmaceuticals. 2022; 15(1):99. https://doi.org/10.3390/ph15010099
Chicago/Turabian StyleMustafa, Ahmed M., Eugenia Mazzara, Doaa Abouelenein, Simone Angeloni, Sonia Nunez, Gianni Sagratini, Víctor López, Marco Cespi, Sauro Vittori, Giovanni Caprioli, and et al. 2022. "Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design" Pharmaceuticals 15, no. 1: 99. https://doi.org/10.3390/ph15010099