Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation
Abstract
:1. Introduction
2. Autophagy
3. Regulation of Autophagosome Formation
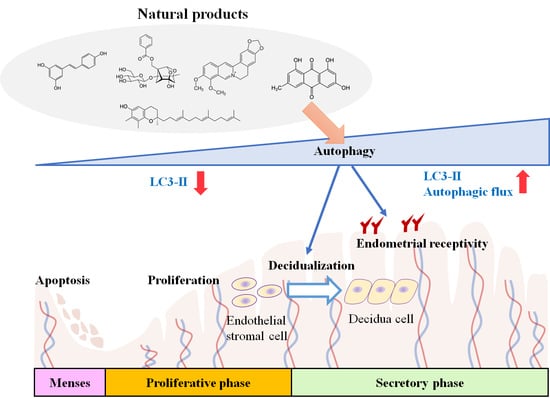
4. Regulation of Embryo Implantation
5. Role of Autophagy in Embryo Implantation
6. Potential Involvement of Autophagic Regulation on the Effect of Natural Products as Embryo Implantation Enhancer
| Classification | Name | Chemical Structure | Biological Action | Autophagy-Related Mode of Action | Effect on Female Reproduction | References |
|---|---|---|---|---|---|---|
| Acetohydroxamic acids | Deferoxamine |  | Antibacterial and heavy metal antagonist | mTOR inhibition; elevation of LC3B expression | Protects endometrial stem cells from oxidative damage | [118,119,136] |
| Alkaloid | Berberine |  | Antioxidant, anticancer, atheroprotective, and immune modulator | Activation of Beclin1; mTOR inhibition | Improves ovulation and endometrial receptivity | [103,137,138,139] |
| Anthraquinone | Emodin |  | Antioxidant, antidiabetic, and anticancer | Elevation of LC3-II expression | Increases the MET of the endometrial stromal cell (decidualization) | [131,132,140,141] |
| Flavonoid | Apigenin |  | Antioxidant and anticancer | mTOR inhibition | Protects the ovary from ischemic/reperfusion and chemotherapy;antagonizes to progesterone; inhibits embryo implantation | [124,142,143,144] |
| Chrysin |  | Antioxidant, neuroprotective, and anticancer | Reduction in LC3-II, Beclin1, and ATG7 levels | Protects the ovary from ischemic/reperfusion | [110,145,146] | |
| Fisetin |  | Antioxidant, neuroprotective, and anticancer | mTOR inhibition; AMPK activation | Reduces PCOS | [111,147,148] | |
| Genistein |  | Antioxidant, anti-inflammatory, and anticancer | Inhibition of PI3K-AKT; enhancement of TFEB activity | Induces implantation failure in neonate mice, but not in puberty | [126,127,128,149] | |
| Kaempferol |  | Antioxidant, neuroprotective, and anticancer | AMPK activation | Increases follicle development;activates progesterone signal; relaxes uterine smooth muscle | [150,151,152,153,154,155] | |
| Quercetin |  | Antioxidant, antiviral, and anticancer | Induction of ATG5 and AMPK activation | Improves follicular development and oocyte quality;inhibits embryo implantation | [129,130,156,157,158] | |
| Wogonin |  | Antioxidant, neuroprotective, anti-inflammation, and anticancer | Induction of ER stress; elevation of LC3-II and Beclin1 levels | Relaxes uterine smooth muscle | [159,160,161] | |
| Lactone | Rapamycin |  | Antibacterial, anticancer, and immunosuppressant | mTOR inhibition | Increases ovarian lifespan | [115,162,163] |
| Brefeldin A |  | Antiviral and protein transport inhibitor | Enhancement of Bip/AKT activation; reduction in AKT phosphorylation | Increases the survival of female germ cells | [104,105,106,107,164] | |
| Lignan | Magnolol |  | Antioxidant, antidiabetic, and anticancer | mTOR inhibition | Inhibits uterine smooth muscle contraction | [165,166,167,168] |
| Polyphenol | Curcumin |  | Antioxidant, antidiabetic, antiallergic, and anticancer | Inhibition of mTOR; enhancement of TFEB activity and LC3 levels | Reduces PCOS and POF;inhibits decidualization | [108,109,125,169,170,171] |
| EGCG, catechin, and epicatechin |  EGCG EGCG | Antioxidant, neuroprotective, anti-inflammation and anticancer | AMPK activation | Enhance ovulation; reduce cyst formation in PCOS | [172,173,174,175,176] | |
| Stilbenoid | Resveratrol |  | Antioxidant, neuroprotective, antidiabetic, and anticancer | AMPK activation | Improves oocyte maturation in aged;increases or decreases decidualization | [133,134,135,177,178,179,180] |
| Terpenoid | Paeoniflorin |  | Antioxidant, anti-inflammatory, neuroprotective, and anticancer | LKB1/AMPK activation | Reduces PCOS;enhances endometrial receptivity | [113,114,120,181,182,183] |
| Ursolic acid |  | Antioxidant, atheroprotective, antidiabetic, and anticancer | mTOR inhibition; elevation of LC3-II, ATG5, and Beclin1 levels | Attenuates POF (hypothetical);suppresses endometrial stromal cell survival | [117,121,184,185,186] | |
| Tocotrienol | γ-Tocotrienol |  | Antioxidant, anti-inflammatory, and anticancer | AMPK activation; elevation of LC3-II, ATG5, and Beclin1 levels | Promotes preimplantation development; improves the quality of embryos | [116,187,188] |
| Xanthonoid | α-Mangostin |  | Antioxidant, neuroprotective, and anticancer | AMPK activation; induction of LC3-II | Protects from ovarian cell death | [112,189,190] |
7. Possible Role of Autophagy on the Effect of Medicinal Herbal Drugs as Embryo Implantation Enhancer
| Name | Active Components | Role in Autophagy | References |
|---|---|---|---|
| BaelanChagsangBang | - | - | [236] |
| Bangdeyun and its component DS147 | - | - | [196,197] |
| Buganshen recipe | - | - | [198] |
| BuShenAnTai recipe | - | - | [201] |
| Bushen Tiaoxue Granules and Kunling Wan | - | - | [202] |
| Dingkun Pill | - | - | [206,207,212] |
| Erbu Zhuyu decoction | - | Increases the Beclin1 and LC3B | [204,208] |
| Gushen’antai pills | - | - | [195] |
| Liuwei Dihuang Granule | - | - | [199] |
| Shoutaiwai recipe | - | - | [200] |
| Tokishakuyakusan(Danggui Shaoyao san) | - | Induces autophagy and mitophagy via increasing PINK1 and LC3 but reducing p62 | [203,214,215] |
| Wenshen Yangxue decoction | - | - | [205,211] |
| Xianziyizhen Recipe | - | - | [237] |
| Yeosin-san | Paeonia lactiflora and Cyperus rotundus | - | [213,238] |
| Yiqixue buganshen recipe | - | - | [198] |
| Zhuyun recipe | - | - | [210] |
| Angelica gigas | Decusirol | Block autophagic flux by suppressing cathepsin C expression | [216,221] |
| Cnidium officinale | - | - | [239] |
| Cyperus rotundus | - | Increases LC3B II/LC3B and Beclin1 | [223,229] |
| Paeonia lactiflora | Paeoniflorin | Induces autophagy via inhibition of AKT/mTOR | [114,225,230] |
| Panax quiquefolius (American Ginseng) | Ginsenoside Rb1 and Rg1 | Induces autophagy via inhibiting AKT/mTOR | [218,240,241] |
| Perilla frutescens var. acuta | Perilaldehyde | Induces autophagy via activating AMPK | [222,228] |
| Rehmannia glutinosa var. purpurea | Catalpol | Induces autophagy via activating AMPK | [224,226,227] |
| Theobroma cacao | - | Induces autophagy via activating sirtuin-1/AMPK signaling | [217,219,220] |
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Jung, M.; Choi, H.; Mun, J.Y. The autophagy research in electron microscopy. Appl. Microsc. 2019, 49, 11. [Google Scholar] [CrossRef] [Green Version]
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992, 119, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Ke, P.Y. Horning cell self-digestion: Autophagy wins the 2016 nobel prize in physiology or medicine. Biomed. J. 2017, 40, 5–8. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Autophagy wins the 2016 nobel prize in physiology or medicine: Breakthroughs in baker′s yeast fuel advances in biomedical research. Proc. Natl. Acad. Sci. USA 2017, 114, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Eskelinen, E.L. Autophagy: Supporting cellular and organismal homeostasis by self-eating. Int. J. Biochem. Cell Biol. 2019, 111, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Poon, A.; Eidelman, D.; Laprise, C.; Hamid, Q. Atg5, autophagy and lung function in asthma. Autophagy 2012, 8, 694–695. [Google Scholar] [CrossRef] [Green Version]
- Pierdominici, M.; Vomero, M.; Barbati, C.; Colasanti, T.; Maselli, A.; Vacirca, D.; Giovannetti, A.; Malorni, W.; Ortona, E. Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J. 2012, 26, 1400–1412. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Pitiakoudis, M.; Gatter, K.C.; Harris, A.L. Beclin 1 over- and underexpression in colorectal cancer: Distinct patterns relate to prognosis and tumour hypoxia. Br. J. Cancer 2010, 103, 1209–1214. [Google Scholar] [CrossRef]
- Trinh, J.; Farrer, M. Advances in the genetics of parkinson disease. Nat. Rev. Neurol. 2013, 9, 445–454. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Hu, W.; Ren, H.; Tian, E.; Zhao, Y.; Lu, Q.; Huang, X.; Yang, P.; Li, X.; et al. C. Elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010, 141, 1042–1055. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in female fertility: A role in oxidative stress and aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, Q.; Wei, D.; Yang, Z.; Du, Y.; Ma, Y. Autophagy in male reproduction. Syst. Biol. Reprod. Med. 2019, 65, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Oh, Y.K.; Choi, D. The role of autophagy in human endometrium. Biol. Reprod. 2012, 86, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, H.; Li, D.; Li, M. Role of endometrial autophagy in physiological and pathophysiological processes. J. Cancer 2019, 10, 3459–3471. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.J.; He, J.L.; Liu, X.Q.; Chen, X.M.; Ding, Y.B.; Tong, C.; Peng, C.; Geng, Y.Q.; Wang, Y.X.; et al. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. 2020, 98, 555–567. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, H.A.; Song, H.; Jun, J.H.; Roh, C.R.; Xie, H.; Dey, S.K.; Lim, H.J. Autophagy regulates embryonic survival during delayed implantation. Endocrinology 2011, 152, 2067–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; Kim, J.S. A review of mechanisms of implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Miravet-Valenciano, J.A.; Rincon-Bertolin, A.; Vilella, F.; Simon, C. Understanding and improving endometrial receptivity. Curr. Opin. Obstet. Gynecol. 2015, 27, 187–192. [Google Scholar] [CrossRef]
- Valdes, C.T.; Schutt, A.; Simon, C. Implantation failure of endometrial origin: It is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil. Steril. 2017, 108, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Ono, M.; Sato, Y.; Imakawa, K.; Iizuka, T.; Kagami, K.; Fujiwara, T.; Horie, A.; Tani, H.; Hattori, A.; et al. Promoting roles of embryonic signals in embryo implantation and placentation in cooperation with endocrine and immune systems. Int. J. Mol. Sci. 2020, 21, 1885. [Google Scholar] [CrossRef] [Green Version]
- Karizbodagh, M.P.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Implantation window and angiogenesis. J. Cell. Biochem. 2017, 118, 4141–4151. [Google Scholar] [CrossRef]
- Davidson, L.M.; Coward, K. Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. Part C Embryo Today 2016, 108, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Rockfield, S.; Taran, N.; Haller, E.; Engelman, R.W.; Flores, I.; Panina-Bordignon, P.; Nanjundan, M. Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis. 2016, 7, e2059. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Kim, H.J.; Choi, D. Differential induction of autophagy by mtor is associated with abnormal apoptosis in ovarian endometriotic cysts. Mol. Hum. Reprod. 2014, 20, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of antioxidant natural products in management of infertility: A review of their medicinal potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Zaid, A.N. Herbal remedies used for the treatment of infertility in males and females by traditional healers in the rural areas of the west bank/palestine. BMC Complement. Altern. Med. 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hyun, M.K.; Kim, H.J.; Kim, D.I. Acupuncture and herbal medicine for female infertility: An overview of systematic reviews. Integr. Med. Res. 2021, 10, 100694. [Google Scholar] [CrossRef]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Raj, S.D.; Fann, D.Y.; Wong, E.; Kennedy, B.K. Natural products as geroprotectors: An autophagy perspective. Med. Res. Rev. 2021, 41, 3118–3155. [Google Scholar] [CrossRef]
- Kim, J.H.; Kismali, G.; Gupta, S.C. Natural products for the prevention and treatment of chronic inflammatory diseases: Integrating traditional medicine into modern chronic diseases care. Evid.-Based Complement. Altern. Med. 2018, 2018, 9837863. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Wiersma, M.; Brundel, B.J.J.M. Role of autophagy in proteostasis: Friend and foe in cardiac diseases. Cells 2018, 7, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323. [Google Scholar] [CrossRef]
- Djajadikerta, A.; Swati, K.; Pavel, M.; Prestil, R.; Ryan, L.; Rubinsztein, D.C. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J. Mol. Biol. 2020, 432, 2799–2821. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.L.; Reith, A.; Seglen, P.O. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin or a lysosomotropic amine. Exp. Cell Res. 1982, 137, 191–201. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. Ampk and tor: The yin and yang of cellular nutrient sensing and growth control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. Ampk and mtor regulate autophagy through direct phosphorylation of ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Hindupur, S.K.; Gonzalez, A.; Hall, M.N. The opposing actions of target of rapamycin and amp-activated protein kinase in cell growth control. Cold Spring Harb. Perspect. Biol. 2015, 7, a019141. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The lkb1-ampk pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing lkb1-ampk pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. Ros signaling under metabolic stress: Cross-talk between ampk and akt pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.; Yoshimori, T.; Tooze, S. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Tina, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. Lk1 induces autophagy by phosphorylating beclin-1 and activating vps34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, J.D. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N.; Hayashi-Nishino, M.; Fukumoto, H.; Omori, H.; Yamamoto, A.; Noda, T.; Yoshimori, T. An atg4b mutant hampers the lipidation of lc3 paralogues and causes defects in autophagosome closure. Mol. Biol. Cell 2008, 19, 4651–4659. [Google Scholar] [CrossRef] [Green Version]
- Weidberg, H.; Shpilka, T.; Shvets, E.; Abada, A.; Shimron, F.; Elazar, Z. Lc3 and gate-16 n termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell 2011, 20, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Baeken, M.W.; Weckmann, K.; Diefenthäler, P.; Schulte, J.; Yusifli, K.; Moosmann, B.; Behl, C.; Hajieva, P. Novel insights into the cellular localization and regulation of the autophagosomal proteins lc3a, lc3b and lc3c. Cells Tissues Organs 2020, 18, 2315. [Google Scholar] [CrossRef]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. Lc3, gabarap and gate16 localize to autophagosomal membrane depending on form-ii formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef] [Green Version]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous lc3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Yim, W.W.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, J.O.; Nah, J.; Jung, Y.K. Molecules and their functions in autophagy. Exp. Mol. Med. 2012, 44, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Sanchez-Alvarez, M.; Lolo, F.N.; Trionfetti, F.; Strippoli, R.; Cordani, M. Autophagy and the lysosomal system in cancer. Cells 2021, 10, 2752. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. Tfeb links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astanina, E.; Bussolino, F.; Doronzo, G. Multifaceted activities of transcription factor eb in cancer onset and progression. Mol. Oncol. 2021, 15, 327–346. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, J.; He, X.; Chen, H.; Wei, T.; Feng, W. Ywha/14-3-3 proteins recognize phosphorylated tfeb by a noncanonical mode for controlling tfeb cytoplasmic localization. Autophagy 2019, 15, 1017–1030. [Google Scholar] [CrossRef]
- Roczniak-Ferguson, A.; Petit, C.S.; Froehlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.C.; Ferguson, S.M. The transcription factor tfeb links mtorc1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 2012, 5, ra42. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, M.; Pal, R.; Nelvagal, H.R.; Lotfi, P.; Stinnett, G.R.; Seymour, M.L.; Chaudhury, A.; Bajaj, L.; Bondar, V.V.; Bremner, L.; et al. Mtorc1-independent tfeb activation via akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat. Commun. 2017, 8, 14338. [Google Scholar] [CrossRef]
- Parr, C.; Carzaniga, R.; Gentleman, S.M.; Van Leuven, F.; Walter, J.; Sastre, M. Glycogen synthase kinase 3 inhibition promotes lysosomal biogenesis and autophagic degradation of the amyloid-beta precursor protein. Mol. Cell. Biol. 2012, 32, 4410–4418. [Google Scholar] [CrossRef] [Green Version]
- Pena-Llopis, S.; Vega-Rubin-de-Celis, S.; Schwartz, J.C.; Wolff, N.C.; Tran, T.A.; Zou, L.; Xie, X.J.; Corey, D.R.; Brugarolas, J. Regulation of tfeb and v-atpases by mtorc1. EMBO J. 2011, 30, 3242–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferron, M.; Settembre, C.; Shimazu, J.; Lacombe, J.; Kato, S.; Rawlings, D.J.; Ballabio, A.; Karsenty, G. A rankl-pkcbeta-tfeb signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 2013, 27, 955–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, X.; Wang, S.; Chen, Y.; Liu, H. Regulation of tfeb activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Malampati, S.; Zeng, Y.; Durairajan, S.S.K.; Yang, C.B.; Tong, B.C.; Iyaswamy, A.; Shang, W.B.; Sreenivasmurthy, S.G.; Zhu, Z.; et al. A small molecule transcription factor eb activator ameliorates beta-amyloid precursor protein and tau pathology in alzheimer’s disease models. Aging Cell 2020, 19, e13069. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [Green Version]
- von Grothusen, C.; Lalitkumar, S.; Boggavarapu, N.R.; Gemzell-Danielsson, K.; Lalitkumar, P.G. Recent advances in understanding endometrial receptivity: Molecular basis and clinical applications. Am. J. Reprod. Immunol. 2014, 72, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, G.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial decidualization: The primary driver of pregnancy health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhry, P.; Asselin, E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines, and growth factors. Endocrinology 2011, 210, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef]
- Charnock-Jones, D.S.; Sharkey, A.; Fenwick, P.; Smith, S.K. Leukaemia inhibitory factor mrna concentration peaks in human endometrium at the time of implantation and the blastocyst contains mrna for the receptor at this time. J. Reprod. Fertil. 1994, 101, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabibzadeh, S.; Kong, Q.; Babaknia, A.; May, L.T. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum. Reprod. 1995, 10, 2793–2799. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Fisher, D.S.; Schust, J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef]
- Binder, N.K.; Evans, J.; Gardner, D.K.; Salamonsen, L.A.; Hannan, N.J. Endometrial signals improve embryo outcome: Functional role of vascular endothelial growth factor isoforms on embryo development and implantation in mice. Hum. Reprod. 2014, 29, 2278–2286. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Chen, C.D.; Chou, C.H.; Wen, W.F.; Tsao, P.N.; Lee, H.; Chen, S.U. Intentional endometrial injury increases embryo implantation potentials through enhanced endometrial angiogenesis. Biol. Reprod. 2018, 100, 381–389. [Google Scholar] [CrossRef]
- Shaulov, T.; Sierra, S.; Sylvestre, C. Recurrent implantation failure in ivf: A canadian fertility and andrology society clinical practice guideline. Reprod. Biomed. Online 2020, 41, 819–833. [Google Scholar] [CrossRef]
- Busnelli, A.; Somigliana, E.; Cirillo, F.; Baggiani, A.; Levi-Setti, P.E. Efficacy of therapies and interventions for repeated embryo implantation failure: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1747. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rouholamin, S.; Rezaeinejad, M.; Maroufizadeh, S.; Sepidarkish, M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103078. [Google Scholar] [CrossRef]
- Cecchino, G.N.; Garcia-Velasco, J.A. Endometrioma, fertility, and assisted reproductive treatments: Connecting the dots. Curr. Opin. Obstet. Gynecol. 2018, 30, 223–228. [Google Scholar] [CrossRef]
- Konrad, L.; Kortum, J.; Nabham, R.; Gronbach, J.; Dietze, R.; Oehmke, F.; Berkes, E.; Tinneberg, H.R. Composition of the stroma in the human endometrium and endometriosis. Reprod. Sci. 2018, 25, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Amălinei, C.; Păvăleanu, I.; Grigoraş, A.; Căruntu, I.D.; Giuşcă, S.E.; Avădănei, E.R.; Lozneanu, L.; Balan, R.A. The endometrial regeneration frontiers: From mechanisms to applications in regenerative medicine. Rom. J. Morphol. Embryol. 2018, 59, 407–425. [Google Scholar]
- Valentijn, A.J.; Palial, K.; Al-Lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. Ssea-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamali, N.; Zal, F.; Mostafavi-Pour, Z.; Samare-Najaf, M.; Poordast, T.; Dehghanian, A. Ameliorative effects of quercetin and metformin and their combination against experimental endometriosis in rats. Reprod. Sci. 2021, 28, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Liu, X.; Guo, S.W. Caloric restriction dramatically stalls lesion growth in mice with induced endometriosis. Reprod. Sci. 2018, 25, 1024–1036. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. In vitro and in vivo effects of mk2206 and chloroquine combination therapy on endometriosis: Autophagy may be required for regrowth of endometriosis. Br. J. Pharmacol. 2018, 175, 1637–1653. [Google Scholar] [CrossRef] [Green Version]
- Zhan, L.; Li, J.; Wei, B. Autophagy in endometriosis: Friend or foe? Biochem. Biophys. Res. Commun. 2018, 495, 60–63. [Google Scholar] [CrossRef]
- Rhee, J.S.; Saben, J.L.; Mayer, A.L.; Schulte, M.B.; Asghar, Z.; Stephens, C.; Chi, M.M.; Moley, K.H. Diet-induced obesity impairs endometrial stromal cell decidualization: A potential role for impaired autophagy. Hum. Reprod. 2016, 31, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Gao, R.; Geng, Y.; Chen, X.; Liu, X.; Zhang, L.; Mu, X.; Ding, Y.; Wang, Y.; He, J. Decreased autophagy was implicated in the decreased apoptosis during decidualization in early pregnant mice. J. Mol. Histol. 2018, 49, 589–597. [Google Scholar] [CrossRef]
- Fimia, G.M.; Stoykova, A.; Romagnoli, A.; Giunta, L.; Di Bartolomeo, S.; Nardacci, R.; Corazzari, M.; Fuoco, C.; Ucar, A.; Schwartz, P.; et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007, 447, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Gan, B.; Peng, X.; Nagy, T.; Alcaraz, A.; Gu, H.; Guan, J.L. Role of fip200 in cardiac and liver development and its regulation of tnfalpha and tsc-mtor signaling pathways. J. Cell Biol. 2006, 175, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oestreich, A.K.; Chadchan, S.; Medvedeva, A.; Lydon, J.P.; Jungheim, E.S.; Moley, K.H.; Kommagani, R. The autophagy protein, fip200 (rb1cc1) mediates progesterone responses governing uterine receptivity and decidualization. Biol. Reprod. 2020, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, A.K.; Chadchan, S.B.; Popli, P.; Medvedeva, A.; Rowen, M.N.; Stephens, C.S.; Xu, R.; Lydon, J.P.; Demayo, F.J.; Jungheim, E.S.; et al. The autophagy gene atg16l1 is necessary for endometrial decidualization. Endocrinology 2020, 161, bqz039. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, A.; Zhang, Y.; Nan, S.; Yin, R.; Lei, Q.; Zhu, H.; Chen, J.; Han, L.; Ding, M.; et al. Essential role of crim1 on endometrial receptivity in goat. Int. J. Mol. Sci. 2021, 22, 5323. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, R.; Zhang, L.; Geng, Y.; Chen, Q.; Chen, X.; Liu, X.; Mu, X.; Ding, Y.; Wang, Y.; et al. Ampk/mtor downregulated autophagy enhances aberrant endometrial decidualization in folate-deficient pregnant mice. J. Cell. Physiol. 2021, 236, 7376–7389. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Duan, Q.; Gao, D.; Wang, Y.; Xue, S.; Li, W.; Lei, M. Zearalenone blocks autophagy flow and induces cell apoptosis during embryo implantation in gilts. Toxicol. Sci. 2020, 175, 126–139. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, K.; Su, H.; Tang, Y.; Wang, H.; Xu, X.; Dong, H. Berberine improves ovulation and endometrial receptivity in polycystic ovary syndrome. Phytomedicine 2021, 91, 153654. [Google Scholar] [CrossRef]
- Ding, W.X.; Ni, H.M.; Gao, W.; Hou, Y.F.; Melan, M.A.; Chen, X.; Stolz, D.B.; Shao, Z.M.; Yin, X.M. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 2007, 282, 4702–4710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.J.; Chen, B.; Feng, X.L.; Ma, H.G.; Sun, L.L.; Feng, Y.M.; Liang, G.J.; Cheng, S.F.; Li, L.; Shen, W. Exposure to brefeldin a promotes initiation of meiosis in murine female germ cells. Reprod. Fertil. Dev. 2015, 2, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Porowska, H.; Paszkiewicz-Gadek, A.; Lemancewicz, D.; Bielawski, T.; Wo Czynski, S. Effect of brefeldin a on membrane localization of muc1 mucin and adhesive properties of cancer cells. Neoplasma 2008, 55, 305–311. [Google Scholar] [PubMed]
- Zhou, L.; Gao, W.; Wang, K.; Huang, Z.; Zhang, L.; Zhang, Z.; Zhou, J.; Nice, E.C.; Huang, C. Brefeldin a inhibits colorectal cancer growth by triggering bip/akt-regulated autophagy. FASEB J. 2019, 33, 5520–5534. [Google Scholar] [CrossRef]
- Chien, Y.J.; Chang, C.Y.; Wu, M.Y.; Chen, C.H.; Horng, Y.S.; Wu, H.C. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: Systematic review with meta-analysis and trial sequential analysis. Nutrients 2021, 13, 684. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Alan, S.; Basak, N. The protective effects of glycyrrhetinic acid and chrysin against ischemia-reperfusion injury in rat ovaries. Biomed. Res. Int. 2018, 2018, 5421308. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Ameliorative effects of fisetin in letrozole-induced rat model of polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2021, 213, 105954. [Google Scholar] [CrossRef]
- Sanchez-Perez, Y.; Morales-Barcenas, R.; Garcia-Cuellar, C.M.; Lopez-Marure, R.; Calderon-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. The alpha-mangostin prevention on cisplatin-induced apoptotic death in llc-pk1 cells is associated to an inhibition of ros production and p53 induction. Chem. Biol. Interact. 2010, 188, 144–150. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, Y.; Wang, X.; Zhu, M. Paeoniflorin attenuates dhea-induced polycystic ovary syndrome via inactivation of tgf-beta1/smads signaling pathway in vivo. Aging 2021, 13, 7084–7095. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xu, B.; Sun, Y.; Lian, M.; Li, Y.; Lin, Y.; Chen, D.; Diao, Y.; Almoiliqy, M.; Wang, L. Paeoniflorin protects against intestinal ischemia/reperfusion by activating lkb1/ampk and promoting autophagy. Pharmacol. Res. 2019, 146, 104308. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Sun, Y.; Li, J.; Zhang, J.; Hao, D.; Liu, W.; Wu, R.; Kong, F.; Peng, X.; Li, J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell 2017, 16, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Min, S.H.; Song, B.S.; Yeon, J.Y.; Kim, J.W.; Bae, J.H.; Park, S.Y.; Lee, Y.H.; Kim, S.U.; Lee, D.S.; et al. Exogenous gamma-tocotrienol promotes preimplantation development and improves the quality of porcine embryos. Reprod. Fertil. Dev. 2015, 27, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. The hypothetical molecular pathways of ursolic acid to attenuate the premature ovarian failure in human. Med. Hypotheses 2021, 153, 110636. [Google Scholar] [CrossRef] [PubMed]
- Shatrova, A.N.; Burova, E.B.; Kharchenko, M.V.; Smirnova, I.S.; Lyublinskaya, O.G.; Nikolsky, N.N.; Borodkina, A.V. Outcomes of deferoxamine action on h2o2-induced growth inhibition and senescence progression of human endometrial stem cells. Int. J. Mol. Sci. 2021, 22, 6035. [Google Scholar] [CrossRef]
- Pullarkat, V.; Meng, Z.; Donohue, C.; Yamamoto, V.N.; Tomassetti, S.; Bhatia, R.; Krishnan, A.; Forman, S.J.; Synold, T.W. Iron chelators induce autophagic cell death in multiple myeloma cells. Leuk. Res. 2014, 38, 988–996. [Google Scholar] [CrossRef]
- Park, H.R.; Choi, H.J.; Kim, B.S.; Chung, T.W.; Kim, K.J.; Joo, J.K.; Ryu, D.; Bae, S.J.; Ha, K.T. Paeoniflorin enhances endometrial receptivity through leukemia inhibitory factor. Biomolecules 2021, 11, 439. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Z.; Chang, Y.; Li, M.; Wu, Q.; Chen, P.; Liang, X. Suppressive effects of ursolic acid on human endometriotic stromal cells survival. Gynecol. Obstet. Investig. 2020, 85, 72–81. [Google Scholar] [CrossRef]
- Radzinsky, V.Y.; Orazov, M.R.; Ivanov, I.I.; Khamoshina, M.B.; Kostin, I.N.; Kavteladze, E.V.; Shustova, V.B. Implantation failures in women with infertility associated endometriosis. Gynecol. Endocrinol. 2019, 35, 27–30. [Google Scholar] [CrossRef]
- Orazov, M.R.; Radzinsky, V.Y.; Ivanov, I.I.; Khamoshina, M.B.; Shustova, V.B. Oocyte quality in women with infertility associated endometriosis. Gynecol. Endocrinol. 2019, 35, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Deppert, W.R.; Pfeifer, D.; Stanzel, S.; Weimer, M.; Hanjalic-Beck, A.; Stein, A.; Strasser, M.; Zahradnik, H.P.; Schaefer, W.R. Potential hazards to embryo implantation: A human endometrial in vitro model to identify unwanted antigestagenic actions of chemicals. Toxicol. Appl. Pharmacol. 2012, 260, 232–240. [Google Scholar] [CrossRef]
- Devi, Y.S.; DeVine, M.; DeKuiper, J.; Ferguson, S.; Fazleabas, A.T. Inhibition of il-6 signaling pathway by curcumin in uterine decidual cells. PLoS ONE 2015, 10, e0125627. [Google Scholar] [CrossRef]
- Li, R.; Zhao, F.; Diao, H.; Xiao, S.; Ye, X. Postweaning dietary genistein exposure advances puberty without significantly affecting early pregnancy in c57bl/6j female mice. Reprod. Toxicol. 2014, 44, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, W.N.; Padilla-Banks, E.; Goulding, E.H.; Lao, S.P.; Newbold, R.R.; Williams, C.J. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol. Reprod. 2009, 80, 425–431. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Padilla-Banks, E.; Suen, A.A.; Royer, L.J.; Zeldin, S.M.; Arora, R.; Williams, C.J. Uterine patterning, endometrial gland development, and implantation failure in mice exposed neonatally to genistein. Environ. Health Perspect. 2020, 128, 37001. [Google Scholar] [CrossRef]
- Shahzad, H.; Giribabu, N.; Karim, K.; Kassim, N.; Muniandy, S.; Kumar, K.E.; Salleh, N. Quercetin interferes with the fluid volume and receptivity development of the uterus in rats during the peri-implantation period. Reprod. Toxicol. 2017, 71, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Bolouki, A.; Zal, F.; Mostafavi-Pour, Z.; Bakhtari, A. Protective effects of quercetin on uterine receptivity markers and blastocyst implantation rate in diabetic pregnant mice. Taiwan J. Obstet. Gynecol. 2020, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xu, Y.; Lu, J.; Zhao, J.; Wei, X.; Liu, P. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ilk. Reprod. Sci. 2016, 23, 1526–1535. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, J.; Li, W.; Chen, X.; Chen, S.; Chen, L. Emodin reverses the epithelial-mesenchymal transition of human endometrial stromal cells by inhibiting ilk/gsk-3beta pathway. Drug Des. Devel. Ther. 2020, 14, 3663–3672. [Google Scholar] [CrossRef]
- Mestre Citrinovitz, A.C.; Langer, L.; Strowitzki, T.; Germeyer, A. Resveratrol enhances decidualization of human endometrial stromal cells. Reproduction 2020, 159, 453–463. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ozaki, R.; Ikemoto, Y.; Murakami, K.; Muter, J.; Matsumoto, A.; Itakura, A.; Brosens, J.J.; Takeda, S. Resveratrol inhibits decidualization by accelerating downregulation of the crabp2-rar pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Ochiai, A.; Brosens, J.J. The actions of resveratrol in decidualizing endometrium: Acceleration or inhibition? Biol. Reprod. 2020, 103, 1152–1156. [Google Scholar] [CrossRef]
- Velasquez, J.; Wray, A.A. Deferoxamine. In Statpearls; FDA: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A.A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive t cells in autoimmune inflammation. J. Cell Mol. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a potential anticancer agent: A comprehensive review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef]
- Xing, L.; Zhou, X.; Li, A.H.; Li, H.J.; He, C.X.; Qin, W.; Zhao, D.; Li, P.Q.; Zhu, L.; Cao, H.L. Atheroprotective effects and molecular mechanism of berberine. Front. Mol. Biosci. 2021, 8, 762673. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Tuorkey, M.; Aggarwal, D.; Parashar, N.C.; Varol, M.; Savla, R.; Kaur, G.; Mittal, S.; Sak, K. Emodin: A metabolite that exhibits anti-neoplastic activities by modulating multiple oncogenic targets. Toxicol. In Vitro 2021, 73, 105142. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, S.; Li, X.; Liu, R. Advances in the study of emodin: An update on pharmacological properties and mechanistic basis. Chin. Med. 2021, 16, 102. [Google Scholar] [CrossRef]
- Talebi, A.; Hayati Roodbari, N.; Reza Sameni, H.; Zarbakhsh, S. Impact of coadministration of apigenin and bone marrow stromal cells on damaged ovaries due to chemotherapy in rat: An experimental study. Int. J. Reprod. Biomed. 2020, 18, 551–560. [Google Scholar] [CrossRef]
- Soyman, Z.; Kelekci, S.; Sal, V.; Sevket, O.; Bayindir, N.; Uzun, H. Effects of apigenin on experimental ischemia/reperfusion injury in the rat ovary. Balkan Med. J. 2017, 34, 444–449. [Google Scholar] [CrossRef]
- Xu, L.; Zaky, M.Y.; Yousuf, W.; Ullah, A.; Abdelbaset, G.R.; Zhang, Y.; Ahmed, O.M.; Liu, S.; Liu, H. The anticancer potential of apigenin via immunoregulation. Curr. Pharm. Des. 2021, 27, 479–489. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective potential of chrysin: Mechanistic insights and therapeutic potential for neurological disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Naureen, H.; Zahid, R.; Youssef, L.; Attar, R.; Xu, B. Cancer chemopreventive role of fisetin: Regulation of cell signaling pathways in different cancers. Pharmacol. Res. 2021, 172, 105784. [Google Scholar] [CrossRef]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. J. Food Biochem. 2021, 45, e13972. [Google Scholar] [CrossRef]
- Revuelta, M.P.; Cantabrana, B.; Hidalgo, A. Mechanisms involved in kaempferol-induced relaxation in rat uterine smooth muscle. Life Sci. 2000, 67, 251–259. [Google Scholar] [CrossRef]
- Toh, M.F.; Mendonca, E.; Eddie, S.L.; Endsley, M.P.; Lantvit, D.D.; Petukhov, P.A.; Burdette, J.E. Kaempferol exhibits progestogenic effects in ovariectomized rats. J. Steroids Horm. Sci. 2014, 5, 136. [Google Scholar]
- Santos, J.M.S.; Monte, A.P.O.; Lins, T.; Barberino, R.S.; Menezes, V.G.; Gouveia, B.B.; Macedo, T.J.S.; Oliveira Junior, J.L.; Donfack, N.J.; Matos, M.H.T. Kaempferol can be used as the single antioxidant in the in vitro culture medium, stimulating sheep secondary follicle development through the phosphatidylinositol 3-kinase signaling pathway. Theriogenology 2019, 136, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.S.; Lins, T.; Barberino, R.S.; Menezes, V.G.; Gouveia, B.B.; Matos, M.H.T. Kaempferol promotes primordial follicle activation through the phosphatidylinositol 3-kinase/protein kinase b signaling pathway and reduces DNA fragmentation of sheep preantral follicles cultured in vitro. Mol. Reprod. Dev. 2019, 86, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Silva Dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The pharmacological action of kaempferol in central nervous system diseases: A review. Front. Pharmacol. 2020, 11, 565700. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.; Ucan, U.; Boyacioglu, M.; Ipek, E.; Akosy, M. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of dietary antioxidants in p53-mediated cancer chemoprevention and tumor suppression. Oxidative Med. Cell. Longev. 2021, 2021, 9924328. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orru, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- Shih, H.C.; Yang, L.L. Relaxant effect induced by wogonin from scutellaria baicalensis on rat isolated uterine smooth muscle. Pharm. Biol. 2012, 50, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Huynh, D.L.; Ngau, T.H.; Nguyen, N.H.; Tran, G.B.; Nguyen, C.T. Potential therapeutic and pharmacological effects of wogonin: An updated review. Mol. Biol. Rep. 2020, 47, 9779–9789. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Abdulmajid Ayatollahi, S.; Kobarfard, F.; Imran, M.; Imran, A.; Custodio, L.; Dolores Lopez, M.; et al. The therapeutic potential of wogonin observed in preclinical studies. Evid.-Based Complement. Altern. Med. 2021, 2021, 9935451. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.J.; Su, Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996, 58, 373–395. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Paek, S.M. Recent synthesis and discovery of brefeldin A analogs. Mar. Drugs 2018, 16, 133. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Chen, H.H.; Ko, C.H.; Lin, Y.R.; Chan, M.H. The mechanism of honokiol-induced and magnolol-induced inhibition on muscle contraction and ca2+ mobilization in rat uterus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 262–269. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A neolignan from the magnolia family for the prevention and treatment of cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, toxicity, bioavailability, and formulation of magnolol: An update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef] [PubMed]
- Szalabska-Rapala, K.; Borymska, W.; Kaczmarczyk-Sedlak, I. Effectiveness of magnolol, a lignan from magnolia bark, in diabetes, its complications and comorbidities-a review. Int. J. Mol. Sci. 2021, 22, 10050. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Liu, S.; Yang, C.; Chen, L.; Tang, F.; Wang, F.; Zhan, L.; Deng, H.; Zhou, W.; et al. Curcumin and its analogs as potential epigenetic modulators: Prevention of diabetes and its complications. Pharmacology 2021, 1–13. [Google Scholar] [CrossRef]
- Memarzia, A.; Saadat, S.; Behrouz, S.; Boskabady, M.H. Curcuma longa and curcumin affect respiratory and allergic disorders, experimental and clinical evidence: A comprehensive and updated review. Biofactors 2021, 47, 311–350. [Google Scholar] [CrossRef] [PubMed]
- Sudhesh Dev, S.; Zainal Abidin, S.A.; Farghadani, R.; Othman, I.; Naidu, R. Receptor tyrosine kinases and their signaling pathways as therapeutic targets of curcumin in cancer. Front. Pharmacol. 2021, 12, 772510. [Google Scholar] [CrossRef]
- Hong, G.; Wu, H.; Ma, S.T.; Su, Z. Catechins from oolong tea improve uterine defects by inhibiting stat3 signaling in polycystic ovary syndrome mice. Chin. Med. 2020, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.M.; Salamt, N.; Zaid, S.S.M.; Mokhtar, M.H. Beneficial effects of green tea catechins on female reproductive disorders: A review. Molecules 2021, 26, 2675. [Google Scholar] [CrossRef]
- Wu, D. Green tea egcg, t-cell function, and t-cell-mediated autoimmune encephalomyelitis. J. Investig. Med. 2016, 64, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective effects of epigallocatechin gallate (egcg) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Fernandes, L.; Cardim-Pires, T.R.; Foguel, D.; Palhano, F.L. Green tea polyphenol epigallocatechin-gallate in amyloid aggregation and neurodegenerative diseases. Front. Neurosci. 2021, 15, 718188. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Sun, A.; Zhao, S.G.; Liu, H.; Ma, S.Y.; Li, M.; Huai, Y.X.; Zhao, H.; Liu, H.B. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Liang, X.; Xu, H.; Liao, Y.; Lu, K.; Lu, S. Effects of resveratrol on in vitro maturation of porcine oocytes and subsequent early embryonic development following somatic cell nuclear transfer. Reprod. Domest. Anim. 2019, 54, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. Npj Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Zhang, Q.; Wei, S.; Huang, C.; Li, Z.; Gao, Y. Paeoniflorin: A monoterpene glycoside from plants of paeoniaceae family with diverse anticancer activities. J. Pharm. Pharmacol. 2020, 72, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.X.; Gong, X.H.; Zhang, H.; Peng, C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Biomed. Pharmacother. 2020, 130, 110505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Feng, S.T.; Wang, Y.T.; Chen, N.H.; Wang, Z.Z.; Zhang, Y. Paeoniflorin: A neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine 2021, 90, 153669. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Yadav, D.K. Therapeutic potential of ursolic acid in cancer and diabetic neuropathy diseases. Int. J. Mol. Sci. 2021, 22, 12162. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Kujacinski, M.; Wicinski, M. Beneficial effects of ursolic acid and its derivatives-focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients 2021, 13, 3900. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.T.; Luk, S.U.; Al-Ejeh, F.; Khanna, K.K. Tocotrienol as a potential anticancer agent. Carcinogenesis 2012, 33, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.J.; Gu, Q.L.; Yang, K.; Ming, X.J.; Wang, J.X. Anticarcinogenic effects of alpha-mangostin: A review. Planta Med. 2017, 83, 188–202. [Google Scholar]
- Yang, A.; Liu, C.; Wu, J.; Kou, X.; Shen, R. A review on alpha-mangostin as a potential multi-target-directed ligand for alzheimer’s disease. Eur. J. Pharmacol. 2021, 897, 173950. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Zhang, Y.; Zhang, Y.; Jia, L.; Zhang, D.; Zhang, J.; Han, Y.; Luo, S. The efficacy of complementary and alternative medicine in the treatment of female infertility. Evid.-Based Complement. Altern. Med. 2021, 2021, 6634309. [Google Scholar] [CrossRef]
- Cao, H.; Han, M.; Ng, E.H.; Wu, X.; Flower, A.; Lewith, G.; Liu, J.P. Can chinese herbal medicine improve outcomes of in vitro fertilization? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e81650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Li, D.; Liu, C.; Ji, X.; Li, R.; Du, X. Effects of chinese herbs combined with in vitro fertilization and embryo transplantation on infertility: A clinical randomized controlled trial. J. Tradit. Chin. Med. 2014, 34, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Li, J.; Li, Y.; Wang, B.; Xie, C.; Xia, Q.; Zhang, Z.; Wang, Y. Chinese herbal medicine for assisted reproduction technology: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e22009. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Song, J.Y.; Zhang, X.X.; Chen, Y.H.; Teng, Y.L.; Liu, H.P.; Deng, T.Y.; Sun, Z.G. Effects of a chinese patent medicine gushen′antai pills on ongoing pregnancy rate of hormone therapy fet cycles: A multi-center, randomized, double-blind, placebo-controlled clinical trial. Front. Endocrinol. 2020, 11, 581719. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, C.; Hu, L.; Li, J. Local immune regulatory effects of bangdeyun on the endometrium of mice with embryo implantation dysfunction during the implantation time. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 372–376. [Google Scholar] [CrossRef]
- Deng, S.R.; Li, J.; Zhang, Z.Q.; Li, B.; Sheng, L.L.; Zeng, J.W.; Liu, Y.P.; An, S.L.; Wu, Y.X. Ds147 improves pregnancy in mice with embryo implantation dysfunction induced by controlled ovarian stimulation. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 573–580. [Google Scholar] [CrossRef]
- Li, H.X.; Guo, X.Y.; Xie, Y.; Ge, M.X.; Yuan, Q.L.; Lin, D.W.; Xiong, L.; Deng, W.M.; Zhang, J.Y. Yiqixue buganshen recipe regulates the expression of integrin alphanubeta3 in the endometrium of controlled ovarian hyperstimulation mice. Chin. J. Integr. Med. 2013, 19, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Wu, H.C.; Sun, Z.G.; Guo, Y.; Shi, L.; Xue, M.Y. Effects of liuwei dihuang granule on the outcomes of in vitro fertilization pre-embryo transfer in infertility women with kidney-yin deficiency syndrome and the proteome expressions in the follicular fluid. Chin. J. Integr. Med. 2014, 20, 503–509. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, J.; Wang, Z.Y.; Yu, X.H.; Wei, B.X.; Wu, X.H. Effects of modified shoutaiwai recipe on integrin beta3 and leukemia-inhibitory factor in endometrium of controlled ovarian hyperstimulation mice during the implantation window. Genet. Mol. Res. 2015, 14, 2970–2977. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Xiao, J.; Song, Y.F.; Ding, J.H.; Tan, X.J.; Song, K.K.; Zhang, M.M. Effect and underlying mechanism of bu-shen-an-tai recipe on ovarian apoptosis in mice with controlled ovarian hyperstimulation implantation dysfunction. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 401–406. [Google Scholar] [CrossRef]
- Lv, B.Y.; Sun, H.Y.; Li, Q.; Zhang, H.L.; Pan, C.S.; Yan, L.; Fan, J.Y.; Li, D.; Han, J.Y. The ameliorating effects of bushen tiaoxue granules and kunling wan on impaired angiogenesis and endometrial receptivity in rats following controlled ovarian hyperstimulation. Microcirculation 2020, 27, e12581. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, K.; Saegusa, Y.; Sekiguchi, K.; Shimizu, T.; Takiyama, M.; Matsumoto, T.; Iizuka, S.; Matsumoto, C.; Motoyama, F. The ameliorating effects of tokishakuyakusan in a rat model of implantation failure involves endometrial gland leukemia inhibitory factor and decidualization. J. Ethnopharmacol. 2021, 265, 113288. [Google Scholar] [CrossRef]
- Yuan, L.; Feng, F.; Mao, Z.; Huang, J.; Liu, Y.; Li, Y.; Jiang, R. Effects of erbuzhuyu decoction combined with acupuncture on endometrial receptivity are associated with the expression of mir-494-3p. Evid.-Based Complement. Altern. Med. 2020, 2020, 9739672. [Google Scholar] [CrossRef]
- Xin, M.; He, J.; Zhang, Y.; Wu, Y.; Yang, W.; Liang, X.; Yin, X. Chinese herbal decoction of wenshen yangxue formula improved fertility and pregnancy rate in mice through pi3k/akt signaling. J. Cell. Biochem. 2019, 120, 3082–3090. [Google Scholar] [CrossRef]
- Song, J.Y.; Gao, D.D.; Cao, X.L.; Xiang, S.; Chen, Y.H.; Teng, Y.L.; Li, X.F.; Liu, H.P.; Wang, F.X.; Zhang, B.; et al. The role of traditional chinese formula ding-kun pill (dkp) in expected poor ovarian response women (poseidon group 4) undergoing in vitro fertilization-embryo transfer: A multicenter, randomized, double-blind, placebo-controlled trial. Front. Endocrinol. 2021, 12, 675997. [Google Scholar] [CrossRef]
- Song, J.; Ma, T.; Liang, Y.; Cao, X.; Sun, Z. Efficacy and safety of dingkun pill for female infertility patients with low prognosis undergoing in vitro fertilization-embryo transfer: Study protocol for a multicenter, double-blind, randomized, placebo-controlled trial. Trials 2020, 21, 550. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Shen, M.; Pan, X.; Xu, X.; Xie, B.; Zheng, H. Influence of erbu zhuyu tang on expressions of endometrial autophagy genes-beclin-1 and lc3b in mice with embryo implantation dysfunction. J. Beijing Univ. Tradit. Chin. Med. 2019, 42, 910–915. [Google Scholar]
- Ding, J.; Tan, X.; Song, K.; Ma, W.; Xiao, J.; Song, Y.; Zhang, M. Bushen huoxue recipe alleviates implantation loss in mice by enhancing estrogen-progesterone signals and promoting decidual angiogenesis through fgf2 during early pregnancy. Front. Pharmacol. 2018, 9, 437. [Google Scholar] [CrossRef]
- Yu, N.; Yang, J.; Yin, T. Extracts from a traditional chinese herbal remedy (zhuyun recipe) improve endometrial receptivity in mice with embryonic implantation dysfunction and ovulation stimulation. J. Ethnopharmacol. 2011, 137, 389–395. [Google Scholar] [CrossRef]
- Xin, M.; He, J.; Yang, W.; Yin, X.; Wang, J. Wenshen yangxue decoction improves endometrial receptivity recovery and promotes endometrial angiogenesis in a rat model. Pharm. Biol. 2018, 56, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, L.; Bao, H.; Xu, Y.; Meng, M.; Qiao, M.; Wang, H.; Kong, S. Traditional chinese medicine dingkun pill facilitates uterine receptivity for implantation in mice. Biol. Reprod. 2019, 101, 695–703. [Google Scholar] [CrossRef]
- Choi, H.J.; Joo, B.S.; Park, M.J.; Park, M.J.; Bae, B.; Kim, B.S.; Park, H.R.; Kim, K.J.; Yang, H.J.; Chung, T.W.; et al. Yeosin-san increases female fertility through inducing uterine receptivity and ovarian function. J. Physiol. Pathol. Korean Med. 2019, 33, 141–150. [Google Scholar] [CrossRef]
- Song, Z.; Luo, D.; Wang, Y.; Zheng, Y.; Chen, P.; Xia, X.; He, C.; Yu, W.; Li, P.; Xiao, C.; et al. Neuroprotective effect of danggui shaoyao san via the mitophagy-apoptosis pathway in a rat model of alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2021, 2021, 3995958. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Chen, H.H.; Tian, J.; Chen, H.J.; Zhu, L.L.; Zhao, P.; Zhang, T. Danggui shaoyao san ameliorates renal fibrosis via regulation of hypoxia and autophagy. Evid.-Based Complement. Altern. Med. 2019, 2019, 2985270. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.E.; Lee, J.E.; Han, Y.H.; Lee, S.I.; Kim, D.K.; Park, S.R.; Yu, S.L.; Kang, J. Decursinol from angelica gigas nakai enhances endometrial receptivity during implantation. BMC Complement. Med. Ther. 2020, 20, 36. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Borovskaya, T.G.; Poluektova, M.E.; Vychuzhanina, A.V.; Shchemerovsma, Y.A.; Kamalova, S.I.; Grgoreva, V.A.; Amoateng, P.; Kukuia, K.E.; Kwapong, A.A.; et al. Reproductive toxicity of theobroma cacao: Increase in survival index, nongenotoxic, and proimplantation potential. J. Toxicol. 2021, 2021, 6114672. [Google Scholar] [CrossRef]
- Belanger, D.; Calder, M.D.; Gianetto-Berruti, A.; Lui, E.M.; Watson, A.J.; Feyles, V. Effects of american ginseng on preimplantation development and pregnancy in mice. Am. J. Chin. Med. 2016, 44, 981–995. [Google Scholar] [CrossRef] [Green Version]
- Arlorio, M.; Bottini, C.; Travaglia, F.; Locatelli, M.; Bordiga, M.; Coisson, J.D.; Martelli, A.; Tessitore, L. Protective activity of theobroma cacao l. Phenolic extract on aml12 and mlp29 liver cells by preventing apoptosis and inducing autophagy. J. Agric. Food Chem. 2009, 57, 10612–10618. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cilleros, D.; Lopez-Oliva, M.E.; Martin, M.A.; Ramos, S. Cocoa ameliorates renal injury in zucker diabetic fatty rats by preventing oxidative stress, apoptosis and inactivation of autophagy. Food Funct. 2019, 10, 7926–7939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, S.I.; Kim, N.; Joo, M.; Lee, K.H.; Lee, M.W.; Jeon, H.J.; Ryu, H.; Kim, J.M.; Sul, J.Y.; et al. Decursin inhibits cell growth and autophagic flux in gastric cancer via suppression of cathepsin c. Am. J. Cancer Res. 2021, 11, 1304–1320. [Google Scholar] [PubMed]
- Kim, E.Y.; Choi, H.J.; Chung, T.W.; Choi, J.Y.; Kim, H.S.; Jung, Y.S.; Lee, S.O.; Ha, K.T. Water-extracted perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins. J. Pharmacol. Sci. 2016, 131, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Chung, T.W.; Park, M.J.; Jung, Y.S.; Lee, S.O.; Kim, K.J.; Ha, K.T. Water-extracted tubers of cyperus rotundus l. Enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin alphavbeta3 and alphavbeta5. J. Ethnopharmacol. 2017, 208, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Chung, T.-W.; Choi, H.-J.; Ha, K.-T.; Jung, Y.-S.; Lee, S.-O.; Choi, J.-Y.; Kim, H.S.; You, S.; Lee, M.S. Extracts from paeonia lactiflora pallas, rehmannia glutinosa var. Purpurea makino, perilla frutescens var. Acuta kudo may increase the endometrial receptivity through expression of leukemia inhibitory factor and adhesion molecules. J. Tradit. Chin. Med. Sci. 2019, 39, 25. [Google Scholar]
- Choi, H.J.; Chung, T.W.; Park, M.J.; Lee, K.S.; Yoon, Y.; Kim, H.S.; Lee, J.H.; Kwon, S.M.; Lee, S.O.; Kim, K.J.; et al. Paeonia lactiflora enhances the adhesion of trophoblast to the endometrium via induction of leukemia inhibitory factor expression. PLoS ONE 2016, 11, e0148232. [Google Scholar]
- Chen, Y.; Liu, Q.; Shan, Z.; Mi, W.; Zhao, Y.; Li, M.; Wang, B.; Zheng, X.; Feng, W. Catalpol ameliorates podocyte injury by stabilizing cytoskeleton and enhancing autophagy in diabetic nephropathy. Front. Pharmacol. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Ren, H.; Wang, D.; Zhang, L.; Kang, X.; Li, Y.; Zhou, X.; Yuan, G. Catalpol induces autophagy and attenuates liver steatosis in ob/ob and high-fat diet-induced obese mice. Aging 2019, 11, 9461–9477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Feng, Q.; Huang, X.; Wang, X.; Peng, Y.; Zhao, Z.; Liu, Z. Perilaldehyde activates amp-activated protein kinase to suppress the growth of gastric cancer via induction of autophagy. J. Cell. Biochem. 2018, 120, 1716–1725. [Google Scholar] [CrossRef]
- Wang, F.; Song, X.; Ma, S.; Liu, C.; Sun, X.; Wang, X.; Liu, Z.; Liang, D.; Yu, Z. The treatment role of cyperus rotundus l. To triple-negative breast cancer cells. Biosci. Rep. 2019, 39, BSR20190502. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, S.; Mu, S.; Guo, R.; Zhou, L.; Fu, Q. Paeoniflorin attenuates dexamethasone-induced apoptosis of osteoblast cells and promotes bone formation via regulating akt/mtor/autophagy signaling pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 6623464. [Google Scholar] [CrossRef]
- Cao, B.Y.; Yang, Y.; Luo, W.F.; Mao, C.J.; Han, R.; Sun, X.; Cheng, J.; Liu, C.F. Paeoniflorin, a potent natural compound, protects pc12 cells from mpp+ and acidic damage via autophagic pathway. J. Ethnopharmacol. 2010, 131, 122–129. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadzhieva, M.B.; Lutcenko, N.N.; Volodin, I.V.; Morozova, K.V.; Salnikova, L.E. Association of oxidative stress-related genes with idiopathic recurrent miscarriage. Free Radic. Res. 2014, 48, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, J.; Yao, G.; Zhu, Q.; Li, X.; Xu, R.; Zhu, Z.; Zhao, H.; Wang, Y.; Ding, Y.; et al. Deficiency of sirtuin 1 impedes endometrial decidualization in recurrent implantation failure patients. Front. Cell Dev. Biol. 2021, 9, 598364. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wei, W.; Cao, R.; Lu, L.; Liang, S.; Xiong, M.; Zhang, C.; Liang, X.; Ma, Y. Resveratrol alleviates zea-induced decidualization disturbance in human endometrial stromal cells. Ecotoxicol. Environ. Saf. 2021, 207, 111511. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Liu, Q.; Cai, H.; Jeong, H.J.; Kim, S.H.; Kim, D.I.; Lee, J.H. Administration of a herbal formulation enhanced blastocyst implantation via iκb activation in mouse endometrium. Chin. Med. 2020, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, Y.Z.; Zhu, X.J.; Zhang, X.; Zhu, J.Y.; Gu, C.X.; Chen, Y.; Huang, J.L. Effect of xianziyizhen recipe capsule on pgi2-ppardelta signaling pathway in embryo implantation dysfunction mice. Am. J. Reprod. Immunol. 2015, 73, 545–556. [Google Scholar] [CrossRef]
- Koo, J.M.; Yang, M.J.; Kim, B.K.; Yoo, J.E.; Park, J.K.; Yang, H.J.; Joo, J.; Joo, B.S.; Heo, J.D.; Ha, K.T. Acute and repeated toxicological study of anti-inflammatory herbal formula, yeosinsan, in rats. Appl. Sci. 2021, 11, 9325. [Google Scholar] [CrossRef]
- Chung, T.W.; Park, M.; Lee, H.; Kim, K.J.; Kim, C.H.; Choi, H.J.; Ha, K.T. Enhancement of endometrial receptivity by cnidium officinale through expressing lif and integrins. Evid.-Based Complement. Altern. Med. 2019, 2019, 7560631. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Cao, G.; Yang, H.; Zhou, H.; Li, L.; Cao, Z.; Yu, B.; Kou, J. A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of ampk/mtor and jnk pathways. J. Neurosci. Res. 2014, 92, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, Y.; Wang, T.; Wang, X.; He, J.; Xu, J.; Wu, B.; Li, Y. Ginsenoside rg1 alleviates podocyte emt passage by regulating akt/gsk3 beta/beta-catenin pathway by restoring autophagic activity. Evid.-Based Complement. Altern. Med. 2020, 2020, 1903627. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Li, L.; Tang, L.Y.; Leung, P.C. Safety evaluation of commonly used chinese herbal medicines during pregnancy in mice. Hum. Reprod. 2012, 27, 2448–2456. [Google Scholar] [CrossRef] [Green Version]
- Dosoky, N.S.; Setzer, W.N. Maternal reproductive toxicity of some essential oils and their constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Oskouei Shirvan, Z.; Etemad, L.; Zafari, R.; Moallem, S.A.; Vahdati-Mashhadian, N.; Hosseinzadeh, H. Teratogenic effect of lippia citriodora leaves aqueous extract in mice. Avicenna J. Phytomed. 2016, 6, 175–180. [Google Scholar]
- Li, L.; Tang, L.Y.; Man, G.C.; Yeung, B.H.; Lau, C.B.; Leung, P.C.; Wang, C.C. Potential reproductive toxicity of largehead atractylodes rhizome, the most commonly used chinese medicine for threatened miscarriage. Hum. Reprod. 2011, 26, 3280–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Xu, L.; Deng, B.; Leng, J.; Tang, N.; Zhao, L.C.; Zhou, H.H.; Zhao, Z.Z.; Yang, Z.J.; Xiao, T.T.; et al. The potential impact of radix paeoniae alba in embryonic development of mice. Phytother. Res. 2017, 31, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Akah, P.A.; Nworu, C.S. Efficacy and safety assessment of t. Angelica herbal tonic, a phytomedicinal product popularly used in nigeria. Evid.-Based Complement. Altern. Med. 2011, 2011, 123036. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, J.S.; Cho, K.J.; Moon, K.N.; Kim, S.Y.; Han, B.; Kim, Y.S.; Jeong, E.J.; Chung, M.K.; Yu, W.J. Developmental and reproductive toxicity assessment in rats with kgc-hj3, korean red ginseng with angelica gigas and deer antlers. J. Ginseng Res. 2019, 43, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jang, J.Y.; Park, D.; Yon, J.M.; Baek, I.J.; Hwang, B.Y.; Nam, S.Y.; Yun, Y.W.; Kim, K.Y.; Joo, S.S.; et al. Korean red ginseng extract does not cause embryo-fetal death or abnormalities in mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 78–85. [Google Scholar] [CrossRef]
- Ratno Budiarto, B.; Chan, W.H. Oxidative stresses-mediated apoptotic effects of ginsenoside rb1 on pre- and post-implantation mouse embryos in vitro and in vivo. Environ. Toxicol. 2017, 32, 1990–2003. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Corsetti, G. Natural compounds and autophagy: Allies against neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Maekawa, T. Nano delivery of natural substances as prospective autophagy modulators in glioblastoma. Nanomedicine 2020, 29, 102270. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Bhaskar, S.; Panda, A.K. Inorganic nanoparticles for natural product delivery: A review. Environ. Chem. Lett. 2020, 18, 2107–2118. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Cho, M.; Do, Y.; Park, J.-K.; Bae, S.-J.; Joo, J.; Ha, K.-T. Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharmaceuticals 2022, 15, 53. https://doi.org/10.3390/ph15010053
Park H, Cho M, Do Y, Park J-K, Bae S-J, Joo J, Ha K-T. Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharmaceuticals. 2022; 15(1):53. https://doi.org/10.3390/ph15010053
Chicago/Turabian StylePark, Hyerin, Minkyoung Cho, Yoonju Do, Jang-Kyung Park, Sung-Jin Bae, Jongkil Joo, and Ki-Tae Ha. 2022. "Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation" Pharmaceuticals 15, no. 1: 53. https://doi.org/10.3390/ph15010053