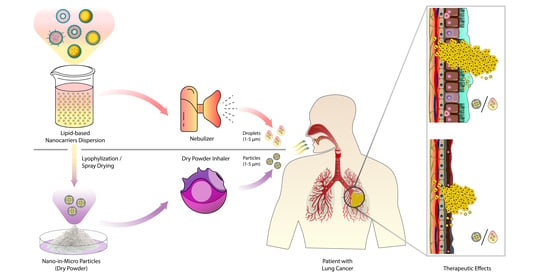
Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update
Abstract
:1. Introduction
2. Methodology
3. Inhalable Anticancer Therapy via Lipid-Based Nanocarriers: Main Advantages and Critical Challenges
4. Physicochemical Considerations, Passive, and Active Targeting For Efficient Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers
5. Devices for the Pulmonary Drug Delivery of Anticancer Drug-Loaded Lipid-Based Nanocarriers
6. Inhalable, Anticancer Drug-Loaded Lipid-Based Nanocarriers
6.1. Liposomes
6.2. Nanoemulsions
6.3. Solid-Lipid Nanoparticles (SLNs)
6.4. Nanostructured Lipid Carriers (NLCs)
6.5. Miscellaneous Inhaled Lipid-Based Nanocarriers
7. Inhalable Anticancer Drug-Loaded Lipid-Based Nanocarriers in Clinical Trials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Global Cancer Observatory. Cancer Fact Sheets. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 23 April 2021).
- American Cancer Society. Key Statistics for Lung Cancer. Available online: https://www.cancer.org/content/cancer/en/cancer/lung-cancer/about/key-statistics.html (accessed on 23 April 2021).
- National Cancer Institute. The Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 23 April 2021).
- World Health Organization. International Agency for Research on Cancer. The Global Cancer Observatory (GCO). Cancer Tomorrow (Incidence). Available online: http://gco.iarc.fr/tomorrow/graphic-line?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0#collapse-group-1-1 (accessed on 23 April 2021).
- World Health Organization. International Agency for Research on Cancer. The Global Cancer Observatory (GCO). Cancer Tomorrow (Mortality). Available online: http://gco.iarc.fr/tomorrow/graphic-line?type=1&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0#collapse-group-1-1 (accessed on 23 April 2021).
- Lovly, L.H.C.M. Neoplasms of the lung. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Dennis, A.S.F., Kasper, L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Butler, S.K. Lung cancer. In Applied Therapeutics: The Clinical Use of Drugs, 11th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; on behalf of the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- American Cancer Society. Treating Non-Small Cell Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell.html (accessed on 23 April 2021).
- American Cancer Society. Surgery for Small Cell Lung Cancer. Available online: https://www.cancer.org/cancer/lung-cancer/treating-small-cell/surgery.html (accessed on 23 April 2021).
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharm. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonciar, D.; Mocan, T.; Matea, C.T.; Zdrehus, C.; Mosteanu, O.; Mocan, L.; Pop, T. Nanotechnology in metastatic cancer treatment: Current achievements and future research trends. J. Cancer 2019, 10, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer and Nanotechnology: Treatment and Therapy. Available online: https://www.cancer.gov/nano/cancer-nanotechnology/treatment (accessed on 22 December 2019).
- Amararathna, M.; Goralski, K.; Hoskin, D.W.; Rupasinghe, H.P.V. Pulmonary nano-drug delivery systems for lung cancer: Current knowledge and prospects. J. Lung Health Dis. 2019, 3, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef]
- Garmany, T.H.; Moxley, M.A.; White, F.V.; Dean, M.; Hull, W.M.; Whitsett, J.A.; Nogee, L.M.; Hamvas, A. Surfactant Composition and Function in Patients with ABCA3 Mutations. Pediatric Res. 2006, 59, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Kwok, P.C.L.; Chan, H.-K. Advances in Pulmonary Drug Delivery, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Williams, R.O.; Taft, D.R.; McConville, J.T. Advanced Drug Formulation Design to Optimize Therapeutic Outcomes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Mangal, S.; Gao, W.; Li, T.; Zhou, Q. Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol. Sin. 2017, 38, 782. [Google Scholar] [CrossRef]
- Haque, S.; Feeney, O.; Meeusen, E.; Boyd, B.J.; McIntosh, M.P.; Pouton, C.W.; Whittaker, M.; Kaminskas, L.M. Local inflammation alters the lung disposition of a drug loaded pegylated liposome after pulmonary dosing to rats. J. Control. Release 2019, 307, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; White, D.; Imondi, A.R.; Placke, M.E.; Vail, D.M.; Kris, M.G. Development of inhalational agents for oncologic use. J. Clin. Oncol. 2001, 19, 1839–1847. [Google Scholar] [CrossRef]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int J. Nanomed. 2012, 7, 1551–1572. [Google Scholar] [CrossRef] [Green Version]
- Videira, M.A.; Botelho, M.F.; Santos, A.C.; Gouveia, L.F.; Pedroso de Lima, J.J.; Almeida, A.J. lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target. 2002, 10, 607–613. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Wittgen, B.P.; Kunst, P.W.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Inhalation SLIT Cisplatin (Liposomal) for the Treatment of Osteosarcoma Metastatic to the Lung. Available online: https://clinicaltrials.gov/ct2/show/NCT00102531 (accessed on 25 June 2021).
- Lemarie, E.; Vecellio, L.; Hureaux, J.; Prunier, C.; Valat, C.; Grimbert, D.; Boidron-Celle, M.; Giraudeau, B.; le Pape, A.; Pichon, E.; et al. Aerosolized gemcitabine in patients with carcinoma of the lung: Feasibility and safety study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investig. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Sapalidis, K.; Zarogoulidis, P.; Sardeli, C.; Koulouris, C.; Giannakidis, D.; Pavlidis, E.; Katsaounis, A.; Michalopoulos, N.; Mantalobas, S.; et al. Inhaled cisplatin for NSCLC: Facts and results. Int. J. Mol. Sci. 2019, 20, 2005. [Google Scholar] [CrossRef] [Green Version]
- Dabbagh, A.; Abu Kasim, N.H.; Yeong, C.H.; Wong, T.W.; Abdul Rahman, N. Critical parameters for particle-based pulmonary delivery of chemotherapeutics. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruge, C.A.; Kirch, J.; Lehr, C.-M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers—Therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Olsson, B.; Bondesson, E.; Borgström, L.; Edsbäcker, S.; Eirefelt, S.; Ekelund, K.; Gustavsson, L.; Hegelund-Myrbäck, T. Pulmonary drug metabolism, clearance, and absorption. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 21–50. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Zhu, X.; Yang, Y.; Liu, Q.; Zhao, D.; Lu, Y.; Wen, J.; Chen, X.; Li, N. Formulation and characterization of spray-dried powders containing vincristine-liposomes for pulmonary delivery and its pharmacokinetic evaluation from in vitro and in vivo. J. Pharm. Sci. 2019, 108, 3348–3358. [Google Scholar] [CrossRef]
- Rosière, R.; Van Woensel, M.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Van Antwerpen, P.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Carvalho, S.R.; McConville, J.T. Formulations for pulmonary administration of anticancer agents to treat lung malignancies. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 61–80. [Google Scholar] [CrossRef]
- Yang, W.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—A current review. Int. J. Pharm. 2008, 356, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J. Nanomed. 2008, 3, 1–19. [Google Scholar]
- El-Sherbiny, I.M.; Villanueva, D.G.; Herrera, D.; Smyth, H.D.C. Overcoming lung clearance mechanisms for controlled release drug delivery. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 101–126. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., 3rd. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Mitragotri, S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Ashitate, Y.; Lee, J.H.; Kim, S.H.; Matsui, A.; Insin, N.; Bawendi, M.G.; Semmler-Behnke, M.; Frangioni, J.V.; Tsuda, A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 2010, 28, 1300–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Hirsjärvi, S.; Passirani, C.; Benoit, J.P. Passive and active tumour targeting with nanocarriers. Curr. Drug Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Yan, W.; Leung, S.S.; To, K.K. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine 2020, 15, 303–318. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, D.; Scalia, S.; Vogel, R.; Wheate, N.; Salama, R.O.; Young, P.M.; Traini, D.; Chrzanowski, W. Magnetised thermo responsive lipid vehicles for targeted and controlled lung drug delivery. Pharm. Res. 2012, 29, 2456–2467. [Google Scholar] [CrossRef] [PubMed]
- Wauthoz, N.; Rosière, R.; Amighi, K. Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin. Drug Deliv. 2020, 1–22. [Google Scholar] [CrossRef]
- Rosière, R.; Amighi, K.; Wauthoz, N. Nanomedicine-based inhalation treatments for lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Academic Press: Cambridge, MA, USA, 2019; pp. 249–268. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.T.; Tang, P.; Leung, S.S.; Chan, J.G.; Chan, H.K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Garrastazu Pereira, G.; Lawson, A.J.; Buttini, F.; Sonvico, F. Loco-regional administration of nanomedicines for the treatment of lung cancer. Drug Deliv. 2016, 23, 2881–2896. [Google Scholar] [CrossRef] [PubMed]
- Longest, W.; Spence, B.; Hindle, M. Devices for improved delivery of nebulized pharmaceutical aerosols to the lungs. J. Aerosol Med. Pulm. Drug Deliv. 2019, 32, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef] [Green Version]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Cipolla, D.; Gonda, I.; Chan, H.K. Liposomal formulations for inhalation. Ther. Deliv. 2013, 4, 1047–1072. [Google Scholar] [CrossRef]
- Hureaux, J.; Lagarce, F.; Gagnadoux, F.; Vecellio, L.; Clavreul, A.; Roger, E.; Kempf, M.; Racineux, J.L.; Diot, P.; Benoit, J.P.; et al. Lipid nanocapsules: Ready-to-use nanovectors for the aerosol delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2009, 73, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Anabousi, S.; Kleemann, E.; Bakowsky, U.; Kissel, T.; Schmehl, T.; Gessler, T.; Seeger, W.; Lehr, C.M.; Ehrhardt, C. Effect of PEGylation on the stability of liposomes during nebulisation and in lung surfactant. J. Nanosci. Nanotechnol. 2006, 6, 3010–3016. [Google Scholar] [CrossRef]
- Wauthoz, N.; Amighi, K. Phospholipids in pulmonary drug delivery. Eur. J. Lipid Sci. Technol. 2014, 116, 1114–1128. [Google Scholar] [CrossRef]
- ElKasabgy, N.A.; Adel, I.M.; Elmeligy, M.F. Respiratory tract: Structure and attractions for drug delivery using dry powder inhalers. AAPS PharmSciTech 2020, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.; Saeed, H.; Sayed, O.M.; Kharshoum, R.M.; Salem, H.F. Proniosomal microcarriers: Impact of constituents on the physicochemical properties of proniosomes as a new approach to enhance inhalation efficiency of dry powder inhalers. AAPS PharmSciTech 2020, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Development and evaluation of paclitaxel and curcumin dry powder for inhalation lung cancer treatment. Pharmaceutics 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Bartos, C.; Farkas, Á.; Szabó-Révész, P.; Ambrus, R. Formulation and in vitro and in silico characterization of “nano-in-micro” dry powder inhalers containing meloxicam. Pharmaceutics 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bothiraja, C.; Mali, A.; Kamble, R. Investigation of sorafenib tosylate loaded liposomal dry powder inhaler for the treatment of non-small cell lung cancer. Part. Sci. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Satari, N.; Taymouri, S.; Varshosaz, J.; Rostami, M.; Mirian, M. Preparation and evaluation of inhalable dry powder containing glucosamine-conjugated gefitinib SLNs for lung cancer therapy. Drug Dev. Ind. Pharm. 2020, 46, 1265–1277. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, Y.; Liu, Q.; Lu, Y.; Xing, H.; Lu, X.; Yang, Y.; Xu, J.; Li, N.; Zhao, D.; et al. Inhalable dry powder prepared from folic acid-conjugated docetaxel liposomes alters pharmacodynamic and pharmacokinetic properties relevant to lung cancer chemotherapy. Pulm. Pharm. Ther. 2019, 55, 50–61. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.-K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef]
- Weers, J.; Clark, A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm. Res. 2017, 34, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Weers, J.G.; Dhand, R. The confusing world of dry powder inhalers: It is all about inspiratory pressures, not inspiratory flow rates. J. Aerosol Med. Pulm. Drug Deliv. 2019, 33, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wu, L.; Chan, H.-K.; Watanabe, W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 1–21. [Google Scholar] [CrossRef]
- Bohr, A.; Water, J.; Beck-Broichsitter, M.; Yang, M. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr. Pharm. Des. 2015, 21, 5829–5844. [Google Scholar] [CrossRef] [PubMed]
- Umerska, A.; Mugheirbi, N.A.; Kasprzak, A.; Saulnier, P.; Tajber, L. Carbohydrate-based Trojan microparticles as carriers for pulmonary delivery of lipid nanocapsules using dry powder inhalation. Powder Technol. 2020, 364, 507–521. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Anton, N.; Jakhmola, A.; Vandamme, T.F. Trojan microparticles for drug delivery. Pharmaceutics 2012, 4, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, H.M.; Freag, M.S.; Elzoghby, A.O. Solid lipid nanoparticle-based drug delivery for lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Academic Press: Cambridge, MA, USA, 2019; pp. 95–121. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free dpi properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- El-Gendy, N.; Berkland, C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm. Res. 2009, 26, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Varshosaz, J.; Hassanzadeh, F.; Mardani, A.; Rostami, M. Feasibility of haloperidol-anchored albumin nanoparticles loaded with doxorubicin as dry powder inhaler for pulmonary delivery. Pharm. Dev. Technol. 2015, 20, 183–196. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Ta, T.M.C.; Zhao, M.; Messaddeq, N.; Vandamme, T.F. Microencapsulation of nanoemulsions: Novel Trojan particles for bioactive lipid molecule delivery. Int. J. Nanomed. 2011, 6, 1313–1325. [Google Scholar] [CrossRef] [Green Version]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Krasia-Christoforou, T. Electrohydrodynamic methods for the development of pulmonary drug delivery systems. Eur. J. Pharm. Sci. 2018, 113, 29–40. [Google Scholar] [CrossRef]
- Ely, L.; Roa, W.; Finlay, W.H.; Löbenberg, R. Effervescent dry powder for respiratory drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Kaur, K.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015, 445, 219–230. [Google Scholar] [CrossRef]
- Roa, W.H.; Azarmi, S.; Al-Hallak, M.H.; Finlay, W.H.; Magliocco, A.M.; Löbenberg, R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J. Control. Release 2011, 150, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallak, M.H.; Sarfraz, M.K.; Azarmi, S.; Roa, W.H.; Finlay, W.H.; Rouleau, C.; Löbenberg, R. Distribution of effervescent inhalable nanoparticles after pulmonary delivery: An in vivo study. Ther. Deliv. 2012, 3, 725–734. [Google Scholar] [CrossRef]
- Kumar, R. Lipid-based nanoparticles for drug-delivery systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar] [CrossRef]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs--a review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for poorly water-soluble anticancer drugs—Barriers of translation and solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, B.C.; Palei, N.N.; Surendran, V.; Dinda, S.C.; Rajangam, J.; Deb, J.; Sahoo, B.M. Lipid based nanoparticles: Current strategies for brain tumor targeting. Curr. Nanomater. 2019, 4, 84–100. [Google Scholar] [CrossRef]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.; Tummala, S.; Madhunapantula, S.V. Lipid-based nanocarriers for breast cancer treatment—Comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhuang, B.; Zhang, G.; Li, M.; Jin, Y. Pulmonary delivery of cationic liposomal hydroxycamptothecin and 5-aminolevulinic acid for chemo-sonodynamic therapy of metastatic lung cancer. Int. J. Pharm. 2021, 601, 120572. [Google Scholar] [CrossRef] [PubMed]
- Loira-Pastoriza, C.; Vanvarenberg, K.; Ucakar, B.; Machado Franco, M.; Staub, A.; Lemaire, M.; Renauld, J.-C.; Vanbever, R. Encapsulation of a CpG oligonucleotide in cationic liposomes enhances its local antitumor activity following pulmonary delivery in a murine model of metastatic lung cancer. Int. J. Pharm. 2021, 600, 120504. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Du, L.; Zeng, J.; Yao, T.; Jin, Y. Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int. J. Pharm. 2020, 578, 119177. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Abdelrahim, M.E.A.; Maged, A.; Abdelkhalek, A.A.; Abdelmoteleb, A.M.M.; Elkasabgy, N.A. Design and characterization of spray-dried proliposomes for the pulmonary delivery of curcumin. Int. J. Nanomed. 2021, 16, 2667–2687. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Yuan, N.; Zhang, X.; Cao, Y.; Jiang, X.; Zhao, S.; Feng, Y.; Fan, Y.; Lu, Z.; Gao, H. Contrast-enhanced computerized tomography combined with a targeted nanoparticle contrast agent for screening for early-phase non-small cell lung cancer. Exp. Ther. Med. 2017, 14, 5063–5068. [Google Scholar] [CrossRef]
- Tagami, T.; Kubota, M.; Ozeki, T. Effective remote loading of doxorubicin into DPPC/poloxamer 188 hybrid liposome to retain thermosensitive property and the assessment of carrier-based acute cytotoxicity for pulmonary administration. J. Pharm. Sci. 2015, 104, 3824–3832. [Google Scholar] [CrossRef]
- Mainelis, G.; Seshadri, S.; Garbuzenko, O.B.; Han, T.; Wang, Z.; Minko, T. Characterization and application of a nose-only exposure chamber for inhalation delivery of liposomal drugs and nucleic acids to mice. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, M.M.; Radomska, A.; Gobbo, O.L.; Bakowsky, U.; Radomski, M.W.; Ehrhardt, C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Zhang, X.; Wong, K.H.; Chen, H.; Liu, Q.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int. J. Nanomed. 2019, 14, 2879–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hamzawy, M.A.; Abo-Youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, M.; Pandya, T.; Gandhi, R.; Patel, S.; Mashru, R.; Misra, A.; Tandel, H. Inhalable liposomal dry powder of gemcitabine-HCl: Formulation, in vitro characterization and in vivo studies. Int. J. Pharm. 2015, 496, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, R.; Li, M.; Bao, J.; Chen, Y.; Ge, Y.; Jin, Y. Comparative study of intratracheal and oral gefitinib for the treatment of primary lung cancer. Eur. J. Pharm. Sci. 2020, 149, 105352. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Ngan, C.L.; Ahmad, H.; Abdulmalek, E.; Masarudin, M.J.; Abdul Rahman, M.B. Excipient selection and aerodynamic characterization of nebulized lipid-based nanoemulsion loaded with docetaxel for lung cancer treatment. Drug Deliv. Transl. Res. 2019, 9, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Asmawi, A.A.; Salim, N.; Abdulmalek, E.; Abdul Rahman, M.B. Modeling the effect of composition on formation of aerosolized nanoemulsion system encapsulating docetaxel and curcumin using D-optimal mixture experimental design. Int. J. Mol. Sci. 2020, 21, 4357. [Google Scholar] [CrossRef] [PubMed]
- Al Ayoub, Y.; Gopalan, R.C.; Najafzadeh, M.; Mohammad, M.A.; Anderson, D.; Paradkar, A.; Assi, K.H. Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. Int. J. Pharm. 2019, 557, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbain, N.H.; Basri, M.; Salim, N.; Wui, W.T.; Abdul Rahman, M.B. Development and characterization of aerosol nanoemulsion system encapsulating low water soluble quercetin for lung cancer treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salim, N.; Masoumi, H.R.F.; Wong, T.W.; Basri, M.; Abdul Rahman, M.B. In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv. Transl. Res. 2019, 9, 497–507. [Google Scholar] [CrossRef]
- Nassimi, M.; Schleh, C.; Lauenstein, H.; Hussein, R.; Hoymann, H.; Koch, W.; Pohlmann, G.A.; Krug, N.; Sewald, K.; Rittinghausen, S. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010, 75, 107–116. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Barar, J.; Aghanejad, A.; Saei, A.A.; Nemati, E.; Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev. Ind. Pharm. 2017, 43, 1244–1253. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Ding, W. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Pharmazie 2010, 65, 585–587. [Google Scholar] [PubMed]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA Porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: Novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv. Healthc. Mater. 2019, 8, 1900965. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted antitumor activity of myricetin against lung carcinoma via nanoencapsulated phospholipid complex in respirable microparticles. Pharm. Res. 2020, 37, 82. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Chougule, M.B.; Patlolla, R.; Wang, G.; Singh, M. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm. Res. 2013, 30, 1435–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Kuzmov, A.; Shah, M.; Garbuzenko, O.B.; Minko, T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control. Release 2013, 171, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: Development and fate upon in vitro deposition on the human epithelial airway barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saimi, M.N.I.; Salim, N.; Ahmad, N.; Abdulmalek, E.; Abdul Rahman, M.B. Aerosolized niosome formulation containing gemcitabine and cisplatin for lung cancer treatment: Optimization, characterization and in vitro evaluation. Pharmaceutics 2021, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Pandey, R.S.; Madan, J.; Jain, U.K. Inhalable cationic niosomes of curcumin enhanced drug delivery and apoptosis in lung cancer cells. Indian J. Pharm. Educ. Res. 2016, 50. [Google Scholar] [CrossRef]
- Osama, H.; Sayed, O.M.; Hussein, R.R.S.; Abdelrahim, M.; Elberry, A. Design, optimization, characterization, and in vivo evaluation of sterosomes as a carrier of metformin for treatment of lung cancer. J. Liposome Res. 2020, 30, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, IN26–IN27. [Google Scholar] [CrossRef]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as potential drug carrier systems for drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Paranjpe, M.; Muller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Chatterjee, B.; Mandal, U.K.; Sengupta, P.; Tekade, R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 2017, 23, 2504–2531. [Google Scholar] [CrossRef] [PubMed]
- Jideani, Y.M.a.V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers, Science and Technology Behind Nanoemulsions; IntechOpen: London, UK, 2018. [Google Scholar]
- Nguyen, T.T.L.; Anton, N.; Vandamme, T.F. Oral pellets loaded with nanoemulsions. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–230. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.; González, C.; Maestro, A.; Solè, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Gorain, B.; Chatterjee, B.; Madheswaran, T.; Md, S.; Mak, K.K.; Tambuwala, M.; Chourasia, M.K.; Kesharwani, P. Nanoemulsions as effective carriers for the treatment of lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–247. [Google Scholar] [CrossRef]
- Ngan, C.L.; Asmawi, A.A. Lipid-based pulmonary delivery system: A review and future considerations of formulation strategies and limitations. Drug Deliv. Transl. Res. 2018, 8, 1527–1544. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef]
- Agrawal, N.; Maddikeri, G.L.; Pandit, A.B. Sustained release formulations of citronella oil nanoemulsion using cavitational techniques. Ultrason. Sonochem. 2017, 36, 367–374. [Google Scholar] [CrossRef]
- Tiwari, S.; Tan, Y.-M.; Amiji, M. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J. Biomed. Nanotechnol. 2006, 2, 217–224. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.; Chan, L.W.; Wong, T.W. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Deliv. 2017, 24, 1631–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 02, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Nesamony, J.; Shah, I.S.; Kalra, A.; Jung, R. Nebulized oil-in-water nanoemulsion mists for pulmonary delivery: Development, physico-chemical characterization and in vitro evaluation. Drug Dev. Ind. Pharm. 2014, 40, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Krahn, C.L.; Raffin, R.P.; Santos, G.S.; Queiroga, L.B.; Cavalcanti, R.L.; Serpa, P.; Dallegrave, E.; Mayorga, P.E.; Pohlmann, A.R.; Natalini, C.C.; et al. Isoflurane-loaded nanoemulsion prepared by high-pressure homogenization: Investigation of stability and dose reduction in general anesthesia. J. Biomed. Nanotechnol. 2012, 8, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.; York, P.; Chrystyn, H.; Clark, B.J. Evaluation of a nanoemulsion-based formulation for respiratory delivery of budesonide by nebulizers. AAPS PharmSciTech 2010, 11, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhu, L.; Liu, B.; Du, L.; Jia, X.; Han, L.; Jin, Y. Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf. B Biointerfaces 2016, 141, 408–416. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Al-Haideri, M.; Seo, T.; Carpentier, Y.A.; Deckelbaum, R.J. Effects of particle size on blood clearance and tissue uptake of lipid emulsions with different triglyceride compositions. JPEN J. Parenter. Enter. Nutr. 2003, 27, 58–64. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 717–728. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef] [Green Version]
- Dammak, I.; Sobral, P.J.d.A.; Aquino, A.; Neves, M.A.d.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Renne, R.A.; Wehner, A.P.; Greenspan, B.; Deford, H.; Ragan, H.A.; Westerberg, R.; Buschbom, R.; Burger, G.; Hayes, A.W.; Suber, R.; et al. 2-week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal. Toxicol. 2008, 4, 95–111. [Google Scholar] [CrossRef]
- Terakosolphan, W. Pharmacokinetic-Modifying Effects of Glycerol in Inhaled Medicines. Ph.D. Thesis, King’s College, London, UK, 2019. [Google Scholar]
- Darquenne, C. Aerosol deposition in the human lung in reduced gravity. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Abbasi, S.; Amini, M.A.; Amani, A. Investigation of factors affecting aerodynamic performance of nebulized nanoemulsion. Iran. J. Pharm Res. 2016, 15, 687–693. [Google Scholar] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Ganta, S.; Singh, A.; Rawal, Y.; Cacaccio, J.; Patel, N.R.; Kulkarni, P.; Ferris, C.F.; Amiji, M.M.; Coleman, T.P. Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer. Drug Deliv. 2016, 23, 968–980. [Google Scholar] [CrossRef]
- Afzal, S.M.; Shareef, M.Z.; Kishan, V. Transferrin tagged lipid nanoemulsion of docetaxel for enhanced tumor targeting. J. Drug Deliv. Sci. Technol. 2016, 36, 175–182. [Google Scholar] [CrossRef]
- Sajid, M.; Cameotra, S.S.; Khan, M.S.A.; Ahmad, I. Chapter 23—Nanoparticle-based delivery of phytomedicines: Challenges and opportunities. In New Look to Phytomedicine; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 597–623. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Composition and structure. In Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–22. [Google Scholar]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Haque, S.; Whittaker, M.; McIntosh, M.P.; Pouton, C.W.; Phipps, S.; Kaminskas, L.M. A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats. Eur. J. Pharm. Biopharm. 2018, 125, 1–12. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Wang, W.; Fu, F.; Wang, W.; Dang, S.; Li, C.; Ma, C.; Zhang, X.; Zhao, Z.; et al. Relationship between particle size and lung retention time of intact solid lipid nanoparticle suspensions after pulmonary delivery. J. Control. Release 2020, 325, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S131–S155. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Amin, S.; Rahman, M.; Ahmad, M.Z.; Rizvi, M.A.; Kamal, M.A.; Ahmad, F.J. Nanotechnology-based inhalation treatments for lung cancer: State of the art. Nanotechnol. Sci. Appl. 2015, 8, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, H.; Hao, J.; Li, B.; Li, M.; Xiuwen, W. Lung cancer combination therapy: Co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2016, 23, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current development of siRNA bioconjugates: From research to the clinic. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.F.; Grimmett, M.E.; Domalewski, C.J.; Cui, H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1586. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A Thermostable, flexible RNA vaccine delivery platform for pandemic response. bioRxiv 2021, 1–26. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: A detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J. Nanomater. 2012, 2012, 358782. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, J.; Pinheiro, M.; Drasler, B.; Septiadi, D.; Petri-Fink, A.; Santos, S.G.; Rothen-Rutishauser, B.; Reis, S. Lipid nanoparticles biocompatibility and cellular uptake in a 3D human lung model. Nanomedicine 2020, 15, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbuzenko, O.B.; Kbah, N.; Kuzmov, A.; Pogrebnyak, N.; Pozharov, V.; Minko, T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J. Control. Release 2019, 296, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Vinas, M.; Fleischer, A.; Palomino Lago, E.; Bachiller, D.; Pedraz, J. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016, 498, 263–273. [Google Scholar] [CrossRef]
- Sans-Serramitjana, E.; Jorba, M.; Fusté, E.; Pedraz, J.L.; Vinuesa, T.; Viñas, M. Free and nanoencapsulated tobramycin: Effects on planktonic and biofilm forms of pseudomonas. Microorganisms 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakunlu, R.I.; Wang, Y.; Tsao, W.; Pozharov, V.; Cook, T.J.; Minko, T. Enhancement of the Efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Nov. Multicompon. Deliv. Syst. 2004, 64, 6214–6224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef] [Green Version]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Saad, M.; Pozharov, V.P.; Reuhl, K.R.; Mainelis, G.; Minko, T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 10737–10742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hida, T.; Kozaki, K.-i.; Muramatsu, H.; Masuda, A.; Shimizu, S.; Mitsudomi, T.; Sugiura, T.; Ogawa, M.; Takahashi, T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin. Cancer Res. 2000, 6, 2006–2011. [Google Scholar]
- Shaik, M.S.; Chatterjee, A.; Jackson, T.; Singh, M. Enhancement of antitumor activity of docetaxel by celecoxib in lung tumors. Int. J. Cancer 2006, 118, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Abou Assi, R.; Abdulbaqi, I.M.; Seok Ming, T.; Siok Yee, C.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and solid self-emulsifying drug delivery systems (SEDDs) as carriers for the oral delivery of azithromycin: Optimization, in vitro characterization and stability assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Le Han, Z.Q.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic combination therapy of lung cancer using paclitaxel-and triptolide-coloaded lipid–polymer hybrid nanoparticles. Drug Des. Dev. Ther. 2018, 12, 3199. [Google Scholar] [CrossRef] [Green Version]
- Yugui, F.; Wang, H.; Sun, D.; Zhang, X. Nasopharyngeal cancer combination chemoradiation therapy based on folic acid modified, gefitinib and yttrium 90 co-loaded, core-shell structured lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2019, 114, 108820. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, Z.; Li, C.; Duan, G.; Wang, K.; Li, Q.; Tao, T. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed. Pharmacother. 2018, 106, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, B.; Fang, C.; Pei, Y. Stealth PEG-PHDCA niosomes: Effects of chain length of PEG and particle size on niosomes surface properties, in vitro drug release, phagocytic uptake, in vivo pharmacokinetics and antitumor activity. J. Pharm. Sci. 2006, 95, 1873–1887. [Google Scholar] [CrossRef]
- Tangri, P.; Khurana, S. Niosomes: Formulation and evaluation. Int. J. Biopharm. 2011, 2229, 7499. [Google Scholar]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharm. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Gajra, B.; Dalwadi, C.; Patel, R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using Box Behnken design. DARU J. Pharm. Sci. 2015, 23, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.K.; Fan, J.; Kim, S.; Bezouglaia, O.; Fartash, A.; Wu, B.M.; Aghaloo, T.; Lee, M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Control. Release 2015, 217, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Doroudgar, M.; Lafleur, M.; Wu, B.M.; Aghaloo, T.; Lee, M. Design and characterization of a therapeutic non-phospholipid liposomal nanocarrier with osteoinductive characteristics to promote bone formation. ACS Nano 2017, 11, 8055–8063. [Google Scholar] [CrossRef] [PubMed]
- ClinicaTtrials.gov. Phase 2 Study of Inhaled Lipid Cisplatin in Pulmonary Recurrent Osteosarcoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01650090 (accessed on 26 June 2021).
- ClinicalTrials.gov. Aerosol L9-NC and Temozolomide in Ewing’s Sarcoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00492141 (accessed on 26 June 2021).
- ClinicalTrials.gov. Phase II Study of Aerosolized Liposomal 9-Nitro-20 (S)- Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/record/NCT00249990 (accessed on 26 June 2021).
- ClinicalTrials.gov. Pharmacology Study of Aerosolized Liposomal 9-Nitro-20 (S)-Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/NCT00250016 (accessed on 25 June 2021).
- ClinicalTrials.gov. Study of Aerosolized Liposomal 9-Nitro-20 (S)- Camptothecin (L9NC). Available online: https://clinicaltrials.gov/ct2/show/NCT00250068 (accessed on 26 June 2021).
- ClinicalTrials.gov. Aerosolized Liposomal Camptothecin in Patients with Metastatic or Recurrent Cancer of the Endometrium or the Lung. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00277082 (accessed on 26 June 2021).
- ClinicalTrials.gov. Pharmacology Study of Aerosolized Liposomal. Available online: https://clinicaltrials.gov/ct2/show/NCT00250120 (accessed on 26 June 2021).


| Drug/Agent | Composition | Aim | Targeting Moiety/ Strategy | Form | Delivery Method/ Device | Cell line/Species | Main Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|
| HC & 5-Aminolevulinic acid | SPC, cholesterol, & octadecylamine | Chemo-sonodynamic therapy for metastatic LC | Cationic liposomes | Liquid | Intratracheal/Insufflator (IA-EC, Penn-Century, Inc., USA) | Formulation evaluation on metastatic LC-bearing mice: Female Balb/c mice (19–21g). | Synergistic effect of the inhaled chemotherapy and sonodynamic therapy led to improved apoptosis of cancer cells | [104] |
| CpG & Poly I:C | DOTAP & DPPC | To locally deliver immunotherapy against LC | N/A | Liquid | Intratracheal instillation | Tumor growth evaluation using murine B16F10 model of metastatic LC, Specific-pathogen-free female C57BL/6 Nrj mice (age, 6–8 weeks). | Delayed tumor growth caused via both agents. Inhalation of the CpG was superior to its intraperitoneal injection in slowing the growth of lung metastases with enhanced antitumor activities. | [105] |
| Paclitaxel | Soybean lecithin & cholesterol. | The investigation of delivering locally live carriers (paclitaxel-in-liposomes-in-bacteria) to combat LC. | Dry liposomes internalized into bacteria (E. coli or L. casei) | Liquid | Intratracheal instillation | Anti-cancer effects evaluation using male SD rats (180–220 g) | Liposomes in E-coli: highest anticancer effect, with the downregulation of VEGF and HIF-1α and the improvement of cancer cell apoptosis | [106] |
| Curcumin | Lecithin, cholesterol, stearylamine, poloxamer 188, 2-hydroxypropyl-β-cyclodextrin | To overcome the curcumin poor aqueous solubility and oral bioavailability | N/A | DP | Intratracheal instillation | MTT assay on A549 Cell line. Pharmacokinetic studies using Albino rats (220–260 g) | The liposomes formulation surpassed curcumin powder in the rate and extent of lung tissue absorption and mean residence time within the lung tissues. | [107] |
| Curcumin | Soybean lecithin & cholesterol | To evaluate the efficacy of curcumin-loaded liposomes | N/A | DP | Insufflator (DP4M, Penn- Century Inc., USA). | MTT assay On BEAS-2B, A549 cells line. Anti-cancer activity evaluation using male SD rats (190–200g). | Liposomes curcumin dry powder showed higher anticancer effects and selectivity than free form. | [108] |
| Lenvatinib-bound to magnetic iron oxide NP | N/A | To investigate the use of inhaled liposomes encapsulating targeted NP contrast agent (TNCA) for diagnosing purposes | Lenvatinib | Liquid | Atomizer | Tomography studies using C57BL/6 mice both sexes (age, 6–8 weeks). | The sensitivity and accuracy of computerized tomography imaging for the diagnosis of early-stage NSCLC was improved. | [109] |
| Doxorubicin | DPPC, Poloxamer 188 | To formulate thermosensitive doxorubicin-loaded liposomes | Hybrid liposomes | Liquid | Intratracheal administration | WST assay on A549 and Raw 264.7 cells lines. The evaluation of lactate dehydrogenase activity and tumor necrosis factor alpha secretion in cell-free bronchoalveolar lavage fluid using Wistar rats (male, age, 13 weeks). | The formulated liposomes administered via the pulmonary route maybe useful for treating LC. | [110] |
| Doxorubicin & ASO, or siRNA | DOTAP & cholesterol. | To evaluate the use of a nose-only exposure chamber for inhalation and delivery of doxorubicin or nucleic acids | ASO, or siRNA | Liquid | One-jet Collison nebulizer (BGI Inc., Waltham, MA, USA) | Evolution of formulation’s distribution and tumor growth size reduction using Nude nu/nu mice (age, 6–8 weeks). | The developed formulation inhalation resulted in tumor volume reduction of more than 90%, whereas only about 40% reduction was achieved after intravenous injection of the free drug. | [111] |
| Doxorubicin | DSPC, DSPE-PEG2000, DSPE-PEG-COOH, & cholesterol | To investigate the efficacy of active drug targeting via TF receptor-mediated uptake. | TF- PEG liposomes | Liquid | An AeroProbe intracorporeal nebulizing catheter connected to a catheter control unit | Tumor induction evaluation using female athymic Rowett nude (rnu) rats (age, 8–10 weeks). | More animals survived in the TF– liposomes groups than in the other treatment regimes, and their lung tissue generally had fewer and smaller tumors. | [112] |
| Quercetin | SPC, DSPE-PEG2000, cholesterol & DSPE-PEG2000- MAL-T7 conjugate | To augment therapeutic efficacy of quercetin-targeting TF receptors. | T7 (HAIYPRH) peptide | Liquid | Microsprayer® Aerosolizer Pulmonary Aerosol- Kit for Mouse (Penn-Century Inc., PA, USA) | MTT assay on A549 and MRC-5 cells lines. Apoptosis, cell-cycle analysis, cellular uptake, and tumor-spheroid penetration and inhibition studies on A549 cell line. Biodistribution study and therapeutic efficacy using male BALB/c nude mice (age, 7–8 weeks). | The developed formulation significantly enhanced the anticancer activity of the drug and lifespan of mice. | [113] |
| Triptolide | SPC, DSPE-PEG2000, DSPE-PEG2000-MALCPP33 | To explore the pulmonary delivery of dual-ligand modified and triptolide-loaded liposomes modified triptolide-loaded liposomes | Anti-CA IX antibody & CPP33 dual ligands | Liquid | Microsprayer Aerosolizer Pulmonary Aerosol Kit for Mouse Model PAK-MSA | Wound healing, apoptosis, penetration, and cytotoxic damage in 3 D tumor spheroids on A549 cell line. Pharmacokinetic study using male SD rats (250 ± 20 g) | The formulation significantly enhanced the anticancer efficacy of the drug without apparent systemic toxicity. | [114] |
| Docetaxel | PC, cholesterol, DSPE-PEG-FA/DSEP-PEG-COOH/Co-spray | To compare the physicochemical properties, and antitumor activities of different targeted liposomal formulations | Folic acid conjugate | Liquid | Intratracheal administration | MTT assay, cellular uptake, endocytic routes study and metabolism assay on A549 and SPCA1 cells lines. bio-distribution studies using SD rats (180–220 g). | The co-spray drying did change the properties, while tracheal administration of the dry powder provided higher drug exposure at the tumor site without increasing the exposure of other organs | [75] |
| Temozolomide | PC, cholesterol & auric tetrachloride | To investigate the possible therapeutic effects of intratracheal inhalation of the developed liposomes-gold NP | N/A | Liquid | Intratracheal administration using Microsprayer IA-1C system (Penn-Century, Philadelphia, PA, USA) | Study the developed formulation’s effects on lung homogenate MDA, GSH and inflammatory cytokines as well as on serum CYFRA 21-1 and IGF-1 level using male BALB/c mice (22–30 g). | The developed liposomes formulations succeed to improve all biochemical data and histological patterns. | [115] |
| Gemcitabine-HCl | HSPC, DSPG, mPEG2000-DSPE. | To formulate and evaluate gemcitabine-HCl -loaded liposomes | PEG- liposomes | DP | Intratracheal administration | MTT assay and cellular uptake on A549 cell line. Maximum tolerated dose, oedema index, acute toxicity study, and pharmacokinetic studies using Wistar rats (200–220 g). | Better pulmonary pharmacokinetic profile of the loaded formulation with lower toxicity to lung tissues than that of drug solution | [116] |
| Vincristine | SPC, & cholesterol. | To improve efficacy, lung exposure and decrease the clearance of the drug | N/A | DP | Intratracheal administration | MTT assay on MCF-7 and A549 cells lines. Absorption and tissue distribution study male SD rats (250 g). | The developed formulation had improved pharmacokinetic behavior of increased maximum concentration and systemic exposure and decreased elimination half-life in comparison to the free drug. | [41] |
| Gefitinib | SPC & cholesterol. | Comparative study of intratracheally administered of gefitinib- liposomes via intratracheally and orally administered free drug | N/A | DP | Intratracheal administration | Pharmacokinetic and biodistribution study using male SD rats (180–200 g). | Intratracheally administered liposomal powder showed higher in vivo therapeutic effect with reduction of inflammation, weak lung injury, and high apoptosis than intratracheally or administered free drug. | [117] |
| Sorafenib tosylate | Phospholipon 90H® & cholesterol | To enhance the physicochemical properties of sorafenib tosylate | N/A | DP | Revolizer device (Cipla Inc.) | NA | The loaded formulation offered biphasic release pattern, burst release in the first 6 h followed by sustained release up to 72 h | [73] |
| Drug/Agent | Composition | Aim | Targeting Moiety/ Strategy | Form | Delivery Method/ Device | Cell line/Species/Subjects | Main Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|
| NEs | ||||||||
| Docetaxel | PKOE, lauric FA, myristic FA, lecithin, Tween 85®, Span 85®, & glycerol | To select biocompatible excipients and perform aerodynamic characterization of nebulized NEs. | N/A | Liquid | OMRON MicroAIR nebulizer | MTT assay on A549 and MRC-5 cell lines | The NEs characteristics nominated it as potential inhalable carriers for docetaxel. | [118] |
| Docetaxel & Curcumin | PKOE, lauric FA, myristic FA, lecithin, Tween 85®, Span 85®, & glycerol | To formulate and optimize aerosolized NEs encapsulating docetaxel and curcumin | N/A | Liquid | OMRON MicroAIR nebulizer | N/A | The optimized NE offered desirable physicochemical and aerodynamic properties for inhalation therapy. | [119] |
| Curcuminoids | Limonene or oleic acid with Tween 80® & ethanol | To prepare nebulized curcuminoid-loaded NEs | N/A | Liquid | Sidestream jet nebulizer | Comet assay on human lymphocytes cells | Both NEs characteristics surpassed the saline-based suspensions of curcuminoid, with no genotoxicity. | [120] |
| Quercetin | PBE, Tween 80®, lecithin & glycerol | To enhance quercetin solubility and cytotoxic selectivity | N/A | Liquid | OMRON MicroAIR nebulizer | MTT assay on A549 and MRC-5 cell lines | Loaded NEs characteristics and release profile were within the pulmonary delivery selection criteria requirements with stable and selective cytotoxic manners. | [121,122] |
| SLNs | ||||||||
| Blank formulation | Lipid mixture (Softisan® & Phospholipon® 90G) & Solutol® HS15 as surfactant. | To evaluate the short-term toxicity of inhaled blank SLNs | N/A | Liquid | A jet-driven aerosol generator system (nebulizer) | MTT assay, neutral red uptake assay on A549 cell line and WST-1 assay using organotypic lung tissue cultures. Short term safety study on female BALB/c mice (age, 8–12 weeks). | This blank SLNs is suitable for pulmonary drug delivery via inhalation. No in vivo record of upregulation in lactate dehydrogenase and inflammation indicators levels | [123] |
| Erlotinib | Compritol 888 ATO®, Tween 80®, poloxamer 407®. | To get rapid drug deposition in lungs, with improved drug therapeutic efficiency and less systemic side effects | N/A | Liquid & DP | N/A | MTT assay on A549 cell line | The developed loaded SLNs surpassed the free drug with a cumulative drug release profile and significant higher anticancer activity. | [124] |
| Epirubicin | Compritol 888 ATO®, lecithin, poloxamer 188®. | To overcome major side effects including hematological and cardiac toxicity. | N/A | Liquid | Pari inhalier boy nebulizer | Crystal violet cytotoxicity assay on A549 cell line. Pharmacokinetic study using male SD rats, (250 ± 20 g) | The suitable SLNs characteristics offered a decrease in drug loss, with possible ability to deliver the drug into the deep lung, enhanced cytotoxicity and pharmacokinetics (~ 2 folds) | [125] |
| Afatinib & paclitaxel | Stearic acid & poloxamer 188®. | To explorer the co-delivery outcome of those drugs. | N/A | Liquid & DP | In vitro: Turbospin, a single dose powder inhaler device. In vivo: a dry powder insufflator | Growth-inhibitory curves study on H1975 and PC9/G cell lines. Short-term safety evaluation, pharmacokinetic and tissue distribution using male SD rats, (180–220 g) | The SLNs characteristics offered extremely high retention in the induction port for both drugs and with no interaction between them or the excipients. Pharmacokinetically, SLNs offered 96 h of a two-stage release and high lung concentration, with no signs of other critical organs distribution | [126] |
| Paclitaxel | Glyceryl-stearate, cholesterol, vitamin E TPGS & sodium taurocholate. | To targeted deliver poor soluble drug | Folate-PEG/chitosan | Liquid | MicroSprayer Aerosolizer IA-1C (endotracheal route) | Cellular uptake on HeLa (CCL-2), M109-HiFR cell lines. Pharmacokinetic study using female (CD-1 and BALB/c mice. | The coated SLNs entered folate receptor (FR)-expressing HeLa and M109-HiFR cells in vitro, and M109 tumors in vivo after pulmonary delivery. The formulation prolonged the pulmonary exposure to paclitaxel up to 6 h and limited systemic distribution. | [42] |
| Myricetin | Gelucires (G 39/01, 50/13, 44/14) & compritol® | To enhance the nutraceutical solubility, stability, and delivery | NA | DP | N/A | MTT assay, and cellular uptake on A549 cell line. | Gelucire-based SLNs were proved to improve the physiochemical properties, release, and anticancer effects of the drug. | [127] |
| Gefitinib | Lecithin, cholesterol, stearic acid, & PEG2000 | To glucosamine targeted | Glucosamine | DP | N/A | MTT assay, and cellular uptake on A549 cell line. | The satisfactory aerosol formulation cellular uptake study clearly demonstrated that functionalization of SLNs with glucosamine promote the accumulation of SLNs within GLUT1 overexpressing cells | [74] |
| NLCs | ||||||||
| Celecoxib | Compritol®, miglyol®, & sodium taurocholate | Evaluation of anticancer synergetic activity of aerosolized celecoxib-NLCs in combination with IV docetaxel. | N/A | Liquid | Inexpose™ nebulizer | MTT assay on A549 cell line. Tumor size reduction evaluation using nu/nu mice (age, 4–6 weeks). | In vivo study proved the synergetic effects of celecoxib-NLCs inhalation and docetaxel IV. | [128] |
| Paclitaxel | Stearic acid (or glyceryl monostearate) oleic acid, Tween 80®, Tween 20®, or Tween 40®. | To compare the oral paclitaxel solution with the paclitaxel-NLCs inhaled delivery | N/A | DP | DP insufflator | Intracellular uptake assay in Caco-2 cell line. Organ distribution of loaded NLCs using male Wistar rats, 180–200 g. | Inhaled paclitaxel-NLCs showed excellent local delivery and organ selectivity when accumulated mainly in lung and in compared to pure drug solution oral intake | [129] |
| Doxorubicin or paclitaxel | Precirol ATO 5®, squalene, Tween 80® | To provide selective local and targeted inhalation lung delivery. | Synthetic analog LHRH/DSPE-PEG2000 and siRNA | DP | Collision nebulizer connected to nose-only exposure chamber for inhalation | Cellular uptake and the intracellular localization on A549 cell line. Tumor size reduction evaluation using athymic nu/nu mice | The developed inhaled NLCs showed high efficiency and selectivity for tumor-targeted local delivery. | [130] |
| Paclitaxel | Precirol ATO 5®, squalene, Tween 80® | To compare the NLC cytotoxicity and selectivity via I.V. and inhalation routes. | LHRH-PEG2000-siRNA | Liquid | Collison nebulizer | MTT assay on A549, H1781, and H3255 cell lines. NLCs organ distribution and tumor size evaluation using nude mice. | Efficient accumulation and retention of the inhaled NLCs in the mice lungs, with no signs of systematic cytotoxicity compared to I.V. route. | [131] |
| LPHNs | ||||||||
| siRNA | PLCGA & DPPC | To downregulate the genes involved in the pathogenesis of LC through the local siRNA delivery | N/A | Liquid | Vibrating mesh nebulizer | MTT assay on A549 and 16HBE14o cell lines | The developed NLCs offered a peculiar triphasic siRNA release lasting for 5 days, with a prolonged inhibition of ENaC protein expression. | [132] |
| Niosomes | ||||||||
| Gemcitabine & cisplatin | Tween 65®, Span 60®, cholesterol, sodium dodecyl sulfate, glycerol | To develop dual drug inhalable niosomes with efficacy but lower dose, and side effects. | N/A | Liquid | OMRON MicroAIR nebulizer | MTT assay on A549 and MRC5 cell lines | Developed NLCs cytotoxicity reduced against the tested cell lines when compared with free drug. | [133] |
| Curcumin | Span 80®, diethyl ether, with or without cholesterol | To formulate C.-niosomes for effective lung delivery | Cationic niosomes | DP | Nebulizer | MTT assay and cellular uptake on A549 cell line | The cholesterol-containing carriers surpassed the cholesterol-free carriers in terms of antiproliferative effects and a higher endocytosis. | [134] |
| Sterosomes | ||||||||
| Metformin | Cholesterol, stearylamine or myristic acid | To evaluate the safety, tolerability, & pharmacokinetics of inhaled metformin sterosomes | N/A | Liquid | Jet nebulizer | MTT assay on A549 cell line. Clinical study (n = 6, 3 males and 3 females age > 18) | The formulated carriers significantly increased the biological half-life area under the curve, and mean residence time of metformin in all healthy volunteers after inhalation. | [135] |
| Lipid Nanocapsules | ||||||||
| Paclitaxel | Captex 8000®, Lipoid S75-3®, & Solutol® HS 15. | To encapsulate paclitaxel in lipid nanocapsules | N/A | Liquid | Jet, ultrasonic and mesh nebulizers of different brands. | Growth inhibition assay on NCI-H460 human lung cancer cells | LNC dispersions could be made into aerosols by using mesh nebulizers without altering the LNC structure. | [66] |
| Drug | Cisplatin | Cisplatin | 9-nitrocamptothecin |
| NCT Number | N/A * | NCT00102531 | N/A ** |
| Phase | Phase I | Phase Ib/IIa | Phase I |
| Nanocarrier Type | Liposomes | Liposomes | Liposomes |
| Nanocarrier composition | Dipalmitoyl phosphatidylcholine (DPPC) and Cholesterol | Dipalmitoyl phosphatidylcholine (DPPC) and Cholesterol | Dilauroyl phosphatidylcholine (DLPC) |
| Drug dose | 1.5–60 mg/m2 | 24 and 36 mg/m2 | 6.7–26.6 µg/kg/day |
| Study duration | 1 to 4 consecutive days in 3-weeks cycles. | The given dose was administered on a 2-weeks cycles. | The given dose was administered for 1 to 8 weeks followed by a 2-weeks rest cycles. |
| Delivering device | Nebulizer | Nebulizer | Nebulizer |
| Droplet size | 3.7 ± 1.9 µm | 3.7 ± 1.9 µm | 1–3 µm |
| No. of Subjects | 17 | 19 | 25 |
| Type of carcinoma | NSCLC (16) SCLC (1) | High-grade, progressive, or recurrent osteosarcoma in the lungs (secondary LC). | Primary or metastatic LC. |
| Subjects’ gender | F + M | F + M | F + M |
| Age, mean (years) | 41.8–70.7, 56.6 | 13–27, 18 ± 3 | 33–84, 58.5 |
| Main adverse events | Dyspnea, vomiting, nausea, cough, hoarseness, and eosinophilia. | Dyspnea, nausea, cough, and wheezing. | Pharyngitis, fatigue, nausea, vomiting, cough, anemia, neutropenia, anorexia, and skin rash |
| Main findings | A significant reversible (in 94% of the subjects) decrease in forced expiratory volume in 1 s (FEV1) was observed after one cycle. | Most of the adverse events occurred at the higher given dose (36 mg/m2). | A decrease in pulmonary function tests during treatment was noticed. |
| No significant change in the diffusing lung capacity for carbon monoxide. | No significant or long-lasting systematic adverse events were noticed. | No hematological toxicities were noticed. | |
| No dose-limiting toxicity was observed at the maximum delivered dose. | No significant change in the pulmonary function testing parameters. | Inhaled 9NC plasma levels were like those observed after oral ingestion. | |
| No systematic adverse effects of cisplatin were noticed. | Serum concentrations of inhaled cisplatin were lower than those of intravenous cisplatin. | A dose-dependent increment in both C max and AUC values at the two lower doses; 6.7 and 13.3 µg/kg/day, but not at the highest dose. | |
| Only 10–15% of the dose reached the site of action. | Systemic cisplatin exposure was minimal. | Partial remissions were observed in 2 patients with uterine cancer, and stabilization occurred in 3 patients with primary lung cancer. | |
| Very low plasma platinum levels only with the longest repeated inhalations. | No significant difference in cisplatin deposition within the tumors and the surrounding lung tissue. | Higher levels of 9NC were found in the lungs compared to those in the plasma by the end of treatment. | |
| 70% of the subjects showed a stable disease, while 23% of them had a progressive disease. | Two patients had stable disease after 2 cycles, underwent metastasectomy, and remained free from pulmonary recurrence 1 year after initiation of therapy. | The recommended dose for Phase II studies was 13.3 µg/kg/day on a daily 60-min exposure, 5 consecutive days/week for 8 weeks, with a concentration of 9NC of 0.4 mg/mL in the nebulizer. | |
| Reference | [30] | [31,32] | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulbaqi, I.M.; Assi, R.A.; Yaghmur, A.; Darwis, Y.; Mohtar, N.; Parumasivam, T.; Saqallah, F.G.; Wahab, H.A. Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update. Pharmaceuticals 2021, 14, 725. https://doi.org/10.3390/ph14080725
Abdulbaqi IM, Assi RA, Yaghmur A, Darwis Y, Mohtar N, Parumasivam T, Saqallah FG, Wahab HA. Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update. Pharmaceuticals. 2021; 14(8):725. https://doi.org/10.3390/ph14080725
Chicago/Turabian StyleAbdulbaqi, Ibrahim M., Reem Abou Assi, Anan Yaghmur, Yusrida Darwis, Noratiqah Mohtar, Thaigarajan Parumasivam, Fadi G. Saqallah, and Habibah A. Wahab. 2021. "Pulmonary Delivery of Anticancer Drugs via Lipid-Based Nanocarriers for the Treatment of Lung Cancer: An Update" Pharmaceuticals 14, no. 8: 725. https://doi.org/10.3390/ph14080725