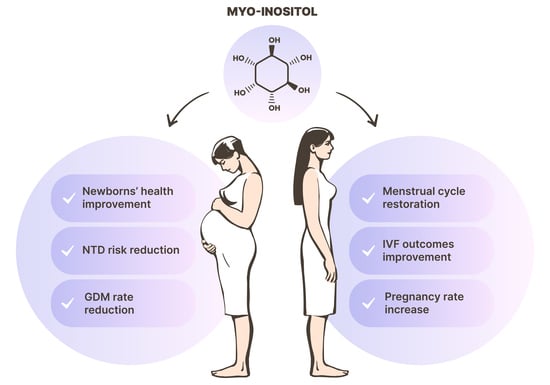
Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation
Abstract
:1. Introduction
1.1. Background
1.2. Inositol in Insulin Signaling
1.3. Inositol in Gonadotropin Signaling
1.4. Inositol in Cellular Motility Phenomena
1.5. Opportunity for Inositol Supplementation
2. First Preclinical Evidence
2.1. Metabolic Maternal Outcomes
2.2. Fetal Outcomes
3. Clinical Applications
3.1. Myo-Inositol in the Pursuit of Pregnancy
3.2. Myo-Inositol in the Prevention of Gestational Diabetes Mellitus
3.3. Myo-Inositol in the Prevention of Neural Tube Defects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gambioli, R.; Forte, G.; Aragona, C.; Bevilacqua, A.; Bizzarri, M.; Unfer, V. The use of D-chiro-Inositol in clinical practice. Eur. Rev. Med. Pharm. Sci. 2021, 25, 438–446. [Google Scholar] [CrossRef]
- Larner, J. D-chiro-inositol-its functional role in insulin action and its deficit in insulin resistance. Int. J. Exp. Diabetes Res. 2002, 3, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharm. 2014, 54, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, I.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim. Biophys. Acta 2004, 1742, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Reiter, E.; Crépieux, P. FSH Receptor Signaling: Complexity of Interactions and Signal Diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two hormones for one receptor: Evolution, biochemistry, actions, and pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Cogram, P.; Hynes, A.; Dunlevy, L.P.; Greene, N.D.; Copp, A.J. Specific isoforms of protein kinase C are essential for prevention of folate-resistant neural tube defects by inositol. Hum. Mol. Genet. 2004, 13, 7–14. [Google Scholar] [CrossRef]
- Unfer, V.; Dinicola, S.; Laganà, A.S.; Bizzarri, M. Altered ovarian inositol ratios may account for pathological steroidogenesis in PCOS. Int. J. Mol. Sci. 2020, 21, 7157. [Google Scholar] [CrossRef]
- National Institutes of Health. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/gestational (accessed on 15 January 2021).
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Goel, M.; Azev, V.N.; d’Alarcao, M. The biological activity of structurally defined inositol glycans. Future Med. Chem. 2009, 1, 95–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.M.A.; Patel, R.; Acevedo-Duncan, M. Protein Kinase C-ζ stimulates colorectal cancer cell carcinogenesis via PKC-ζ/Rac1/Pak1/β-Catenin signaling cascade. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.N.; Batty, I.H.; Prescott, A.R.; Gray, A.; Kular, G.S.; Stewart, H.; Downes, C.P. Inositol phospholipids regulate the guanine-nucleotide-exchange factor Tiam1 by facilitating its binding to the plasma membrane and regulating GDP/GTP exchange on Rac1. Biochem. J. 2004, 382, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Goyal, M.; Sharma, C.; Shekhar, S. Letrozole versus clomiphene citrate for ovulation induction in anovulatory women with polycystic ovarian syndrome: A randomized controlled trial. Int. J. Gynaecol. Obs. 2021, 152, 345–350. [Google Scholar] [CrossRef]
- Mejia, R.B.; Summers, K.M.; Kresowik, J.D.; van Voorhis, B.J. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Jorquera, G.; Echiburú, B.; Crisosto, N.; Sotomayor-Zárate, R.; Maliqueo, M.; Cruz, G. Metformin during Pregnancy: Effects on Offspring Development and Metabolic Function. Front. Pharm. 2020, 11, 653. [Google Scholar] [CrossRef]
- Copp, A.J.; Stanier, P.; Greene, N.D. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013, 12, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, F.; Facchinetti, F.; Ontiveros, A.E.; Roberts, R.P.; Saade, M.M.; Blackwell, S.C.; Sibai, B.M.; Refuerzo, J.S.; Longo, M. The effect of combined inositol supplementation on maternal metabolic profile in pregnancies complicated by metabolic syndrome and obesity. Am. J. Obs. Gynecol. 2016, 215, 503.e1–503.e8. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Alrais, M.; Tamayo, E.H.; Ferrari, F.; Facchinetti, F.; Refuerzo, J.S.; Blackwell, S.C.; Sibai, B.M. Vascular and metabolic profiles in offspring born to pregnant mice with metabolic syndrome treated with inositols. Am. J. Obs. Gynecol. 2019, 220, 279.e1–279.e9. [Google Scholar] [CrossRef]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [CrossRef] [Green Version]
- Gerli, S.; Mignosa, M.; di Renzo, G.C. Effects of inositol on ovarian function and metabolic factors in women with PCOS: A randomized double blind placebo-controlled trial. Eur. Rev. Med. Pharm. Sci. 2003, 7, 151–159. [Google Scholar]
- Costantino, D.; Minozzi, G.; Minozzi, E.; Guaraldi, C. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: A double-blind trial. Eur. Rev. Med. Pharm. Sci. 2009, 13, 105–110. [Google Scholar]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; de Santis, L.; Fusi, F.; Brigante, C.; Marelli, G.; Cino, I.; Redaelli, A.; Ferrari, A. Myo-inositol in patients with polycystic ovary syndrome: A novel method for ovulation induction. Gynecol. Endocrinol. 2007, 23, 700–703. [Google Scholar] [CrossRef]
- Raffone, E.; Rizzo, P.; Benedetto, V. Insulin sensitiser agents alone and in co-treatment with r-FSH for ovulation induction in PCOS women. Gynecol. Endocrinol. 2010, 26, 275–280. [Google Scholar] [CrossRef] [PubMed]
- U-Allah, I.; Sabeen, N.; Iqbal, Q.; Zulfiqar, S.; Wasim, T. Myoinositol in restoring spontaneous ovarian activity in patients with Polycystic Ovarian Syndrome (PCOS). Esculapio 2020, 16, 41–45. [Google Scholar] [CrossRef]
- Pundir, J.; Psaroudakis, D.; Savnur, P.; Bhide, P.; Sabatini, L.; Teede, H.; Coomarasamy, A.; Thangaratinam, S. Inositol treatment of anovulation in women with polycystic ovary syndrome: A meta-analysis of randomised trials. BJOG 2018, 125, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, T.T.; Tam, P.P. A correlation of the outcome of clinical in vitro fertilization with the inositol content and embryotrophic properties of human serum. J. Assist. Reprod. Genet. 1992, 9, 524–530. [Google Scholar] [CrossRef]
- Chiu, T.T.; Rogers, M.S.; Law, E.L.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: Relationship with oocyte quality. Hum. Reprod. 2002, 17, 1591–1596. [Google Scholar] [CrossRef] [Green Version]
- Ravanos, K.; Monastra, G.; Pavlidou, T.; Goudakou, M.; Prapas, N. Can high levels of D-chiro-inositol in follicular fluid exert detrimental effects on blastocyst quality? Eur. Rev. Med. Pharm. Sci. 2017, 21, 5491–5498. [Google Scholar] [CrossRef]
- Lisi, F.; Carfagna, P.; Oliva, M.M.; Rago, R.; Lisi, R.; Poverini, R.; Manna, C.; Vaquero, E.; Caserta, D.; Raparelli, V.; et al. Pretreatment with myo-inositol in non polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: A pilot study. Reprod. Biol. Endocrinol. 2012, 10, 52. [Google Scholar] [CrossRef]
- Caprio, F.; D’Eufemia, M.D.; Trotta, C.; Campitiello, M.R.; Ianniello, R.; Mele, D.; Colacurci, N. Myo-inositol therapy for poor-responders during IVF: A prospective controlled observational trial. J. Ovarian Res. 2015, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laganà, A.S.; Vitagliano, A.; Noventa, M.; Ambrosini, G.; D’Anna, R. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: A systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obs. 2018, 298, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Colazingari, S.; Treglia, M.; Najjar, R.; Bevilacqua, A. The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: Results from a randomized controlled trial. Arch. Gynecol. Obs. 2013, 288, 1405–1411. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, D.; Zhang, Y.; Lin, Y.; Song, J.; Li, S.; Sun, Y. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine 2017, 96, e8842. [Google Scholar] [CrossRef]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruzzetti, F.; Perini, D.; Russo, M.; Bucci, F.; Gadducci, A. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2017, 33, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Shokrpour, M.; Foroozanfard, F.; Afshar Ebrahimi, F.; Vahedpoor, Z.; Aghadavod, E.; Ghaderi, A.; Asemi, Z. Comparison of myo-inositol and metformin on glycemic control, lipid profiles, and gene expression related to insulin and lipid metabolism in women with polycystic ovary syndrome: A randomized controlled clinical trial. Gynecol. Endocrinol. 2019, 35, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, M.; Nigro, M.; Montagnani, M. The putative metabolic role of d-chiro inositol phosphoglycan in human pregnancy and preeclampsia. J. Reprod. Immunol. 2014, 101–102, 140–147. [Google Scholar] [CrossRef]
- Pillai, R.A.; Islam, M.O.; Selvam, P.; Sharma, N.; Chu, A.H.Y.; Watkins, O.C.; Godfrey, K.M.; Lewis, R.M.; Chan, S.Y. Placental Inositol Reduced in Gestational Diabetes as Glucose Alters Inositol Transporters and IMPA1 Enzyme Expression. J. Clin. Endocrinol. Metab. 2021, 106, e875–e890. [Google Scholar] [CrossRef]
- Chu, A.H.Y.; Tint, M.T.; Chang, H.F.; Wong, G.; Yuan, W.L.; Tull, D.; Nijagal, B.; Narayana, V.K.; Meikle, P.J.; Chang, K.T.E.; et al. High placental inositol content associated with suppressed pro-adipogenic effects of maternal glycaemia in offspring: The GUSTO cohort. Int. J. Obes. 2021, 45, 247–257. [Google Scholar] [CrossRef]
- Guo, X.; Guo, S.; Miao, Z.; Li, Z.; Zhang, H. Myo-inositol lowers the risk of developing gestational diabetic mellitus in pregnancies: A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. J. Diabetes Complicat. 2018, 32, 342–348. [Google Scholar] [CrossRef]
- D’Anna, R.; di Benedetto, V.; Rizzo, P.; Raffone, E.; Interdonato, M.L.; Corrado, F.; di Benedetto, A. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol. Endocrinol. 2012, 28, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Matarrelli, B.; Vitacolonna, E.; D’Angelo, M.; Pavone, G.; Mattei, P.A.; Liberati, M.; Celentano, C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2013, 26, 967–972. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: A prospective, randomized, placebo-controlled study. Diabetes Care 2013, 36, 854–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obs. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, A.; Di Benedetto, A.; Petrella, E.; Pintaudi, B.; Corrado, F.; D’Anna, R.; Neri, I.; Facchinetti, F. Myo-inositol may prevent gestational diabetes onset in overweight women: A randomized, controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.; Alibrandi, A.; Di Benedetto, A.; Pintaudi, B.; Corrado, F.; Facchinetti, F.; D’Anna, R. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: A secondary analysis from 3 RCTs. Am. J. Obs. Gynecol. 2018, 219, 300.e1–300.e6. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Corrado, F.; Caruso, S.; di Benedetto, A.; Giunta, L.; Cianci, A.; D’Anna, R. Myo-inositol supplementation to prevent gestational diabetes in overweight non-obese women: Bioelectrical impedance analysis, metabolic aspects, obstetric and neonatal outcomes—A randomized and open-label, placebo-controlled clinical trial. Int. J. Food Sci. Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Celentano, C.; Matarrelli, B.; Pavone, G.; Vitacolonna, E.; Mattei, P.A.; Berghella, V.; Liberati, M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: A randomized controlled trial. J. Matern. Fetal. Neonatal Med. 2018, 33, 743–751. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the prevention of gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obs. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Kandasamy, V.; Subramanian, M.; Rajilarajendran, H.; Ramanujam, S.; Saktivel, S.; Sivaanandam, R. A study on the incidence of neural tube defects in a tertiary care hospital over a period of five years. J. Clin. Diagn. Res. 2015, 9, QC01–QC04. [Google Scholar] [CrossRef]
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina bifida. Nat. Rev. Dis. Primers 2015, 1, 15007. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Available online: https://www.nichd.nih.gov/health/topics/spinabifida/conditioninfo/affected-risk (accessed on 22 January 2021).
- Li, S.; Luo, D.; Yue, H.; Lyu, J.; Yang, Y.; Gao, T.; Liu, Y.; Qin, J.; Wang, X.; Guan, Z.; et al. Neural tube defects: Role of lithium carbonate exposure in embryonic neural development in a murine model. Pediatr. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Facchinetti, F.; Cavalli, P.; Copp, A.J.; D’Anna, R.; Kandaraki, E.; Greene, N.D.E.; Unfer, V. An update on the use of inositols in preventing gestational diabetes mellitus (GDM) and neural tube defects (NTDs). Expert Opin. Drug Metab. Toxicol. 2020, 16, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Oleinik, N.V.; Helke, K.L.; Kistner-Griffin, E.; Krupenko, N.I.; Krupenko, S.A. Rho GTPases RhoA and Rac1 mediate effects of dietary folate on metastatic potential of A549 cancer cells through the control of cofilin phosphorylation. J. Biol. Chem. 2014, 289, 26383–26394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, N.D.; Copp, A.J. Inositol prevents folate-resistant neural tube defects in the mouse. Nat. Med. 1997, 3, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cockroft, D.L.; Brook, F.A.; Copp, A.J. Inositol deficiency increases the susceptibility to neural tube defects of genetically predisposed (curly tail) mouse embryos in vitro. Teratology 1992, 45, 223–232. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.O.; Jung, J.H.; Kim, J.H.; Park, S.H.; Park, J.M.; Kim, E.K.; Suh, P.G.; Kim, H.S. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J. Biol. Chem. 2008, 283, 33969–33974. [Google Scholar] [CrossRef] [Green Version]
- Groenen, P.M.; Peer, P.G.; Wevers, R.A.; Swinkels, D.W.; Franke, B.; Mariman, E.C.; Steegers-Theunissen, R.P. Maternal myo-inositol, glucose, and zinc status is associated with the risk of offspring with spina bifida. Am. J. Obs. Gynecol. 2003, 189, 1713–1719. [Google Scholar] [CrossRef]
- D’Souza, S.W.; Copp, A.J.; Greene, N.D.E.; Glazier, J.D. Maternal Inositol Status and Neural Tube Defects: A Role for the Human Yolk Sac in Embryonic Inositol Delivery? Adv. Nutr. 2021, 12, 212–222. [Google Scholar] [CrossRef]
- Cavalli, P.; Tedoldi, S.; Riboli, B. Inositol supplementation in pregnancies at risk of apparently folate-resistant NTDs. Birth Defects Res. A Clin. Mol. Teratol. 2008, 82, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, P.; Tonni, G.; Grosso, E.; Poggiani, C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.D.; Leung, K.Y.; Gay, V.; Burren, K.; Mills, K.; Chitty, L.S.; Copp, A.J. Inositol for the prevention of neural tube defects: A pilot randomised controlled trial. Br. J. Nutr. 2016, 115, 974–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Study | Patients | Protocol | Findings |
|---|---|---|---|
| Gerli et al. 2003 [22] | 283 PCOS women with oligomenorrhea or amenorrhea | Randomized, placebo-controlled, treatment with 100 mg twice a day for 14 weeks | Ovulation rate: 23% in the treatment group versus 13% in the control group |
| Costantino et al. 2009 [23] | 42 PCOS women with oligomenorrhea, high serum free testosterone, and/or hirsutism | Double-blind, randomized, placebo-controlled, treatment with 2000 mg twice a day for 6 weeks | Ovulation rate: 69.5% in the treatment group versus 21% in the control group Progesterone peak value: 15.1 ng/mL in the treatment group versus 6.6 ng/mL in the control gorup |
| Papaleo et al. 2007 [24] | 25 infertile women showing PCOS as the only apparent cause of infertility | Open-label treatment with 2000 mg twice a day for 6 months | Serum progesterone: 1.8 ± 0.7 ng/mL at baseline versus 10.5 ± 1.8 ng/mL after treatment Percentage of patients with at least one menstrual cycle: 0% at baseline versus 88% after treatment Percentage of patients with regular ovulations: 0% at baseline versus 72% after treatment Percentage of pregnancy achieved during the treatment: 40% |
| Raffone et al. 2010 [25] | 120 anovulatory, infertile PCOS women | Randomized treatment for 6 months with 4000 mg/die myo-inositol versus 1500 mg/die metformin; nonpregnant patients from both groups underwent 37.5 U/die FSH treatment for a maximum of three times | Pregnancy rate after the first treatment: 26.1% in the metformin group versus 28.9% in the myo-inositol group Total pregnancy rate following FSH treatment: 36.6% in the metformin group versus 48.4% in the myo-inositol group |
| Allah et al. 2020 [26] | 140 sub-fertile PCOS women | Open-label treatment with 2000 mg per day for 6 months | Percentage of patients with regular menstrual cycle: 0% at baseline versus 24.3% after three months versus 53.6% after six months Percentage of ovulating patients: 0% at baseline versus 38.6% after three months versus 72.1% after six months |
| Study | Patients | Protocol | Findings |
|---|---|---|---|
| Lisi et al. 2012 [31] | 100 non-PCOS women with basal FSH <10 mUI/mL | Randomized, controlled treatment with 2000 mg twice a day for 3 months | Exogenous FSH required to reach follicular maturation: 2.084 UI in the treatment group versus 2.479 UI in the control group |
| Caprio et al. 2015 [32] | 76 non-PCOS infertile women | Controlled treatment with 4000 mg/day for 3 months | Percentage of metaphase II oocytes: 80.5% in the treatment group versus 66.6% in the control group Ovarian sensitivity index: 1.88 ± 0.81 in the treatment group versus 1.54 ± 0.65 in the control group |
| Study | Patients | Protocol | Findings |
|---|---|---|---|
| D’Anna et al. 2012 [43] | 98 pregnant PCOS women | Retrospective study of women taking 4000 mg/die myo-inositol throughout the whole pregnancy versus 1500 mg/die metformin until pregnancy occurs | GDM incidence: 17.4% in the treatment group versus 54% in the control group |
| Matarrelli et al. 2013 [44] | 73 pregnant women, or intended to become pregnant, with glycemia ≥5.1 mmol/L or 92 mg/dL and ≤7.0 mmol/L or 126 mg/dL | Randomized, double-blind, placebo-controlled treatment with 4000 mg/die for the entire pregnancy | GDM incidence: 6% in the treatment group versus 71% in the control group Need for insulin: 3% in the treatment group versus 21% in the control group Neonatal hypoglycemia: 0% in the treatment group versus 26% in the control group |
| D’Anna et al. 2013 [45] | 197 pregnant women with a parent with type 2 diabetes | Randomized, placebo-controlled treatment with 2000 mg twice per day | GDM incidence: 6% in the treatment group versus 15.3% in the control group Macrosomia cases: 0 in the treatment group versus 7 in the control group Birthweight: 3111 ± 447 g in the treatment group versus 3273 ± 504 g in the control group |
| D’Anna et al. 2015 [46] | 201 pregnant women with BMI ≥ 30 kg/m2 | Randomized, placebo-controlled treatment with 2000 mg twice per day | GDM incidence: 14% in the treatment group versus 33.6% in the control group |
| Santamaria et al. 2016 [47] | 207 women with BMI > 25 and <30 kg/m2 and fasting plasma glucose ≤126 mg/dL and/or glycemia <200 mg/dL | Randomized, placebo-controlled treatment with 2000 mg twice per day from the first trimester to the end of the pregnancy | GDM incidence: 11.6% in the treatment group versus 27.4% in the control group |
| Vitale et al. 2020 [49] | 223 women with BMI > 25 and <30 kg/m2 and fasting plasma glucose ≤126 mg/dL and/or glycemia <200 mg/dL | Randomized, placebo-controlled treatment with 2000 mg twice per day from the first trimester to three weeks after delivery | GDM incidence: 8.2% in the treatment group versus 21.2% in the control group Weight gain: 8.33 ± 2.47 kg in the treatment group versus 9.31 ± 2.66 kg in the control group Total body water in the third trimester: 51.30 ± 4.65 L in the treatment group versus 53.82 ± 4.13 L in the control group |
| Celentano et al. 2018 [50] | 157 nonobese pregnant women with fasting glycemia ≥5.1 mmol/L or 92 mg/dL and <7.0 mmol/L or 126 mg/dL | Randomized, placebo-controlled treatment for the entire pregnancy with 2000 mg myo-inositol twice per day, 500 mg d-chiro-inositol per day, or 13.8 mg d-chiro-inositol and 550 mg myo-inositol twice per day | GDM incidence: 5.1% in the myo-inositol group versus 34.4% in the d-chiro-inositol group versus 38.2% in the combined group versus 61.5% in the control group Neonatal hypoglycemia: 0% in the myo-inositol group versus 15.6% in the d-chiro-inositol group versus 8.8% in the combined treatment group versus 21.1% in the control group |
| Study | Patients | Protocol | Findings |
|---|---|---|---|
| Cavalli et al. 2008 [63] | 3 women with at least one previous pregnancy affected by folate-resistant NTD | Open-label treatment with 500 mg per day from at least two months before and until 60 days after conception | NTD incidence: 0% |
| Cavalli et al. 2011 [64] | 9 women with at least one previous pregnancy affected by folate-resistant NTD | Open-label treatment with 1000 mg per day from at least two months before and until 60 days after conception | NTD incidence: 0% |
| Greene et al. 2016 [65] | 47 randomized and 22 non-randomized women with at least one previous pregnancy affected by NTD | Randomized, double-blind, placebo-controlled treatment with 500 mg twice per day; women who declined randomization decided to take myo-inositol plus folic acid (19 patients), or folic acid only (3 patients) | NTD incidence in randomized patients: 0% in the treatment group versus 5.3% in the control group NTD cases in the non-randomized patients: 0 in the myo-inositol plus folic acid group versus 2 in the folic acid alone group Overall NTD incidence: 0% in the treatment group versus 13.63% in the control group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambioli, R.; Forte, G.; Buzzaccarini, G.; Unfer, V.; Laganà, A.S. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals 2021, 14, 504. https://doi.org/10.3390/ph14060504
Gambioli R, Forte G, Buzzaccarini G, Unfer V, Laganà AS. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals. 2021; 14(6):504. https://doi.org/10.3390/ph14060504
Chicago/Turabian StyleGambioli, Riccardo, Gianpiero Forte, Giovanni Buzzaccarini, Vittorio Unfer, and Antonio Simone Laganà. 2021. "Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation" Pharmaceuticals 14, no. 6: 504. https://doi.org/10.3390/ph14060504