Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity
Abstract
:1. Introduction
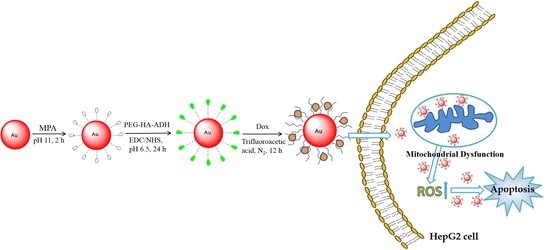
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Cytotoxicity Testing
2.3. Cellular Uptake In Vitro
2.4. Apoptosis Study
2.5. Effect of Dox-Loaded Gold Nanoparticles (AuNPs) on Cell Mitochondrial Membrane Potential
2.6. Change in Intracellular Reactive Oxygen Species (ROS)
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of Au
3.4. Synthesis of Au@MPA
3.5. Synthesis of PEG-HA-ADH
3.6. Synthesis of Au@MPA-PEG-HA-ADH
3.7. Synthesis of Au@MPA-PEG-HA-ADH-Dox
3.8. Synthesis of Au@MPA-PEG-HA-Dox
3.9. Antitumor Activity Assays of AuNPs
3.10. Cellular Uptake of AuNPs
3.11. Early Cell Apoptosis
3.12. Analysis of Mitochondrial Membrane Potential (MMP)
3.13. Measurement of ROS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; et al. Recent Advances in Porphyrin-Based Nanocomposites for Effective Targeted Imaging and Therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Minagawa, Y.; Ueno, H.; Kawai, F.; Murata, T.; Iino, R. Single-Molecule Analysis Reveals Rotational Substeps and Chemo-Mechanical Coupling Scheme of Enterococcus Hirae V1-ATPase. J. Biol. Chem. 2019, 294, 17017–17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerosa, C.; Crisponi, G.; Nurchi, V.M.; Saba, L.; Cappai, R.; Cau, F.; Faa, G.; Van Eyken, P.; Scartozzi, M.; Floris, G.; et al. Gold Nanoparticles: A New Golden Era in Oncology? Pharmaceuticals 2020, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-X.; Cai, Z.-C.; Zhu, B.-J.; Zhang, Z.-Q. The Apoptosis Effect on Liver Cancer Cells of Gold Nanoparticles Modified with Lithocholic Acid. Nanoscale Res. Lett. 2018, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Liu, G.; Zhang, G.; Hu, J.; Liu, S. Engineering Cross-Linkable Plasmonic Vesicles for Synergistic Chemo-Photothermal Therapy Using Orthogonal Light Irradiation. Macromolecules 2018, 51, 8530–8538. [Google Scholar] [CrossRef]
- Cobley, C.M.; Chen, J.Y.; Cho, E.C.; Wang, L.V.; Xia, Y.N. Gold Nanostructures: A Class of Multifunctional Materials for Biomedical Applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.; Rossi, J.J. Targeted Molecular Imaging Using Aptamers in Cancer. Pharmaceuticals 2018, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic Nanoparticles for Cancer Imaging and Therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Zhang, X.; Xi, Z.Q.; Machuki, J.O.; Luo, J.J.; Yang, D.Z.; Li, J.J.; Cai, W.B.; Yang, Y.; Zhang, L.J.; Tian, J.W.; et al. Gold Cube-in-Cube Based Oxygen Nanogenerator: A Theranostic Nanoplatform for Modulating Tumor Microenvironment for Precise Chemo-Phototherapy and Multimodal Imaging. ACS Nano 2019, 13, 5306–5325. [Google Scholar] [CrossRef]
- Gong, N.Q.; Ma, X.W.; Ye, X.X.; Zhou, Q.F.; Chen, X.A.; Tan, X.L.; Yao, S.K.; Huo, S.D.; Zhang, T.B.; Chen, S.Z.; et al. Carbon-Dot-Supported Atomically Dispersed Gold as a Mitochondrial Oxidative Stress Amplifier for Cancer Treatment. Nat. Nanotechnol. 2019, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.G.; Mackeya, M.H.; Mackey, M.A.; Wang, D.S.; Shin, H.J.; Chen, Z.G.; Xiao, H.P.; et al. Efficacy, Long-Term Toxicity, and Mechanistic Studies of Gold Nanorods Photothermal Therapy of Cancer in Xenograft Mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safwat, M.A.; Kandil, B.A.; Elblbesy, M.A.; Soliman, G.M.; Eleraky, N.E. Epigallocatechin-3-Gallate-Loaded Gold Nanoparticles: Preparation and Evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing Mice. Pharmaceuticals 2020, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Zhang, X.B.; Lv, Y.F.; Gong, L.; Wang, R.W.; Zhu, X.Y.; Yang, R.H.; Tan, W.H. Functional DNA-Containing Nanomaterials: Cellular Applications in Biosensing, Imaging, and Targeted Therapy. Acc. Chem. Res. 2014, 47, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-F.; Zhu, B.-J.; Cai, Z.-C.; Wang, C.; Zhao, M.-X. Polyamine-Modified Gold Nanoparticles Readily Adsorb on Cell Membranes for Bioimaging. ACS Omega 2019, 4, 17850–17856. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Gong, Y.; Guo, X.J.; Zhang, P.; Ding, C.-F. Multifunctional Gold Nanoparticle-Based Fluorescence Resonance Energy-Transfer Probe for Target Drug Delivery and Cell Fluorescence Imaging. ACS Appl. Mater. Inter. 2018, 36, 127–136. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Chen, C. Remote Control and Modulation of Cellular Events by Plasmonic Gold Nanoparticles: Implications and Opportunities for Biomedical Applications. ACS Nano 2017, 11, 2403–2409. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified Hyaluronic Acid Based Materials for Biomedical Applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Li, W.; Yi, X.; Liu, X.; Zhang, Z.; Fu, Y.; Gong, T. Hyaluronic Acid Ion-Pairing Nanoparticles for Targeted Tumor Therapy. J. Control. Release 2016, 225, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Babich, O.; Prosekov, A.; Patyukov, N.; Ivanova, S. Future of Chondroprotectors in the Treatment of Degenerative Processes of Connective Tissue. Pharmaceuticals 2020, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-Hyaluronan Synergy Strongly Reduces Intrasynovial Tissue Boundary Friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.B.; Lim, W.Q.; Verma, A.; Chen, H.Z.; Thanabalu, T.; Zhao, Y.L. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, e1803549. [Google Scholar] [CrossRef]
- Harada, H.; Takahashi, M. CD44-Dependent Intracellular and Extracellular Catabolism of Hyaluronic Acid by Hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, C.; Wang, W.; Yang, J.; Wang, H.; Hong, W.; Huang, Y. CD44 Receptor Targeting and Endosomal pH-Sensitive Dual Functional Hyaluronic Acid Micelles for Intracellular Paclitaxel Delivery. Mol. Pharmaceut. 2016, 13, 4209–4221. [Google Scholar] [CrossRef]
- Zhong, Y.N.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.H.; Deng, C.; Zhong, Z.Y.; Haag, R. Hyaluronic Acid-Shelled Acid-Activatable Paclitaxel Prodrug Micelles Effectively Target and Treat CD44-Overexpressing Human Breast Tumor Xenografts in Vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Song, L.; Pan, Z.; Zhang, H.; Li, Y.; Zhang, Y.; Lin, J.; Su, G.; Ye, S.; Xie, L.; Li, Y.; et al. Dually Folate/CD44 Receptor-Targeted Self-Assembled Hyaluronic Acid Nanoparticles for Dual-Drug Delivery and Combination Cancer Therapy. J. Mater. Chem. B 2017, 5, 6835–6846. [Google Scholar] [CrossRef]
- Lengers, I.; Herrmann, F.; Borgne, M.L.; Jose, J. Improved Surface Display of Human Hyal1 and Identification of Testosterone Propionate and Chicoric Acid as New Inhibitors. Pharmaceuticals 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and Delivery Mechanisms of Hyaluronic Acid Used for Topical/Transdermal Delivery-A Review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef] [PubMed]
- Rippe, M.; Cosenza, V.; Auzély-Velty, R. Design of Soft Nanocarriers Combining Hyaluronic Acid with Another Functional Polymer for Cancer Therapy and Other Biomedical Applications. Pharmaceutics 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic Acid and Its Derivatives in Drug Delivery and Imaging: Recent Advances and Challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, S.; Beack, S.; Yang, J.A.; Yun, S.H.; Hahn, S.K. In vivo Real-Time Confocal Microscopy for Target-Specific Delivery of Hyaluronic Acid-Quantum Dot Conjugates. Nanomedicine 2012, 8, 1070–1073. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target Specific and Long-Acting Delivery of Protein, Peptide, and Nucleotide Therapeutics Using Hyaluronic Acid Derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Crosslinking of Hyaluronic Acid with Water-Soluble Carbodiimide. J. Biomed. Mater. Res. 1997, 37, 243–251. [Google Scholar] [CrossRef]
- Jevsevar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of Therapeutic Proteins. Biotechnol. J. 2010, 5, 113–128. [Google Scholar] [CrossRef] [Green Version]
- Wiedenhoeft, T.; Braun, T.; Springer, R.; Teske, M.; Noetzel, E.; Merkel, R.; Csiszár, A. The Basement Membrane in a 3D Breast Acini Model Modulates Delivery and Anti-Proliferative Effects of Liposomal Anthracyclines. Pharmaceuticals 2020, 13, 256. [Google Scholar] [CrossRef]
- Leach, J.B.; Schmidt, C.E. Characterization of Protein Release from Photocrosslinkable Hyaluronic Acid-Polyethylene Glycol Hydrogel Tissue Engineering Scaffolds. Biomaterials 2005, 26, 125–135. [Google Scholar] [CrossRef]
- Yildiz, G.; Aydogmus, Z.; Senkal, F.; Turan, G. Investigation of Curcumin Water Solubility through Emulsifying with Biocompatible Polyethylene Glycol-Based Polymers. Food Anal. Method. 2019, 12, 2129–2138. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network Connectivity, Mechanical Properties and Cell Adhesion for Hyaluronic Acid/PEG Hydrogels. Biomaterials 2011, 32, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Nwhator, S.O.; Umeizudike, K.A.; Sorsa, T. Letter to the Editor: “MMP-8-Responsive Polyethylene Glycol Hydrogel for Intraoral Drug Delivery”. J. Dent. Res. 2019, 98, 1045. [Google Scholar] [CrossRef]
- Yang, C.L.; Wu, T.T.; Qi, Y.; Zhang, Z.P. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein Adsorption is Required for Stealth Effect of Poly(ethylene glycol)- and Poly(phosphoester)-Coated Nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Tan, S.W.; Feng, S.S. Vitamin E TPGS as a Molecular Biomaterial for Drug Delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, J.; Xue, B.; Zhang, C.; Shi, L.; Wu, C.; Su, Y.; Jin, X.; Liu, Y.; Zhu, X. Molecular Insights for the Biological Interactions between Polyethylene Glycol and Cells. Biomaterials 2017, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Zhou, F.F.; Zhang, D.; Chen, Q.; Xing, D. A Graphene Oxide Based Smart Drug Delivery System for Tumor Mitochondria-Targeting Photodynamic Therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef] [Green Version]
- Duhem, N.; Danhier, F.; Preat, V. Vitamin E-Based Nanomedicines for Anti-cancer Drug Delivery. J. Control. Release 2014, 182, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.S.; Ganios, A.M.; Mcburney, D.L.; Dilisio, M.F.; Weiner, S.D.; Horton, W.E., Jr.; Becker, M.L. ECM Production of Primary Human and Bovine Chondrocytes in Hybrid PEG Hydrogels Containing Type I Collagen and Hyaluronic Acid. Biomacromolecules 2012, 13, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stidl, R.; Denne, M.; Goldstine, J.; Kadish, B.; Korakas, K.I.; Turecek, P.L. Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety. Pharmaceuticals 2018, 11, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchakina, O.N.; Ban, H.; Hostetler, B.J.; McKallip, R.J. Inhibition of Hyaluronic Acid Formation Sensitizes Chronic Myelogenous Leukemia to Treatment with Doxorubicin. Glycobiology 2016, 26, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalandon, Y.; Thomas, X.; Hayette, S.; Cayuela, J.M.; Abbal, C.; Huguet, F.; Raffoux, E.; Leguay, T.; Rousselot, P.; Lepretre, S.; et al. Randomized Study of Reduced-Intensity Chemotherapy Combined with Imatinib in Adults with Ph-Positive Acute Lymphoblastic Leukemia. Blood 2015, 125, 3711–3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silber, J.H. Can Dexrazoxane Reduce Myocardial Injury in Anthracycline-Treated Children with Acute Lymphoblastic Leukemia? Nat. Clin. Pract. Oncol. 2004, 1, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, J.W.; Whitney, E.; Cathel, A.; Khan, Y.R.; Mahato, D. Primary Cranial Vault Non-Hodgkin’s Lymphoma Mimicking Meningioma with Positive Angiography. Cureus 2020, 12, e8856. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Möbus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Lübbe, K.; Huober, J.; Klare, P.; Kümmel, S.; Untch, M.; et al. Intense Dose-Dense Epirubicin, Paclitaxel, Cyclophosphamide Versus Weekly Paclitaxel, Liposomal Doxorubicin (Plus Carboplatin in Triple-Negative Breast Cancer) for Neoadjuvant Treatment of High-Risk Early Breast Cancer (GeparOcto-GBG 84): A Randomised Phase III Trial. Eur. J. Cancer 2019, 106, 181–192. [Google Scholar] [PubMed]
- Robledo-Cadena, D.X.; Gallardo-Pérez, J.C.; Dávila-Borja, V.; Pacheco-Velázquez, S.C.; Belmont-Díaz, J.A.; Ralph, S.J.; Blanco-Carpintero, B.A.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells. Pharmaceuticals 2020, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.Y.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. Co-Delivery of Drugs and Plasmid DNA for Cancer Therapy. Adv. Drug Deliv. Rev. 2016, 98, 41–63. [Google Scholar] [CrossRef]
- Hanušová, V.; Boušová, I.; Skálová, L. Possibilities to Increase the Effectiveness of Doxorubicin in Cancer Cells Killing. Drug Metab. Rev. 2011, 43, 540–557. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, Z.; Sun, Z.; Ma, X.; Li, X. Functional Nanoparticles of Tea Polyphenols for Doxorubicin Delivery in Cancer Treatment. J. Mater. Chem. B 2017, 5, 7622–7627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; He, D.; Zhou, Y.; Qin, L. Surface-Modified Nanoerythrocyte Loading DOX for Targeted Liver Cancer Chemotherapy. Mol. Pharmaceut. 2018, 51, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as Heterogeneous Photocatalyst for Facile and Selective Reduction of Nitroarenes to Azo Compounds. J. Am. Chem. Soc. 2018, 140, 12592–12601. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and Near-Infrared Active; Chitosan-Coated Halloysite Nanotubes Loaded with Curcumin-Au Hybrid Nanoparticles for Cancer Drug Delivery. Int. J. Biol. Macromol. 2018, 11, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Avakyan, L.A.; Heinz, M.; Skidanenko, A.V.; Yablunovski, K.A.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; Bugaev, L.A. Insight on Agglomerates of Gold Nanoparticles in Glass Based on Surface Plasmon Resonance Spectrum: Study by Multi-Spheres T-Matrix Method. J. Phys. Condens. Matter. 2018, 30, 045901. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Compounds | IC50 (μg/mL) | |||
|---|---|---|---|---|
| HCT-116 | Hela | HL-7702 | HepG2 | |
| Au | 643.1 ± 2.6 | 667.6 ± 1.9 | 690.9 ± 2.1 | 632.3 ± 3.1 |
| Au@MPA | 513.5 ± 1.5 | 536.5 ± 2.6 | 596.6 ± 3.6 | 497.1 ± 1.4 |
| Au@MPA-PEG-HA-ADH | 325.3 ± 1.1 | 362.1 ± 1.5 | 453.8 ± 1.9 | 329.6 ± 2.5 |
| Au@MPA-PEG-HA-Dox | 183.6 ± 2.3 | 147.2 ± 1.7 | 168.1 ± 2.1 | 169.4 ± 1.3 |
| Au@MPA-PEG-HA-ADH-Dox | 54.6 ± 3.2 | 86.3 ± 2.9 | 103.9 ± 2.0 | 67.3 ± 1.0 |
| Dox | 89.3 ± 1.3 | 91.6 ± 1.6 | 77.9 ± 1.2 | 80.6 ± 2.3 |
| PEG-HA-ADH | 783.2 ± 3.4 | 780.1 ± 1.4 | 806.1 ± 1.8 | 764.3 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.-S.; Ren, B.; Yang, X.; Cai, Z.-C.; Zhao, X.-J.; Zhao, M.-X. Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity. Pharmaceuticals 2021, 14, 101. https://doi.org/10.3390/ph14020101
Li L-S, Ren B, Yang X, Cai Z-C, Zhao X-J, Zhao M-X. Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity. Pharmaceuticals. 2021; 14(2):101. https://doi.org/10.3390/ph14020101
Chicago/Turabian StyleLi, Lin-Song, Bin Ren, Xiaojing Yang, Zhong-Chao Cai, Xue-Jie Zhao, and Mei-Xia Zhao. 2021. "Hyaluronic Acid-Modified and Doxorubicin-Loaded Gold Nanoparticles and Evaluation of Their Bioactivity" Pharmaceuticals 14, no. 2: 101. https://doi.org/10.3390/ph14020101