Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression
Abstract
:1. Introduction
2. Results
2.1. Search Results
2.2. Study, Patient and Treatment Characteristics
2.3. Risk of Bias (ROB)
2.4. Effects on Depression Symptomatology
2.4.1. Endpoint Data
2.4.2. Change Scores
2.5. Effects on Inflammatory Status
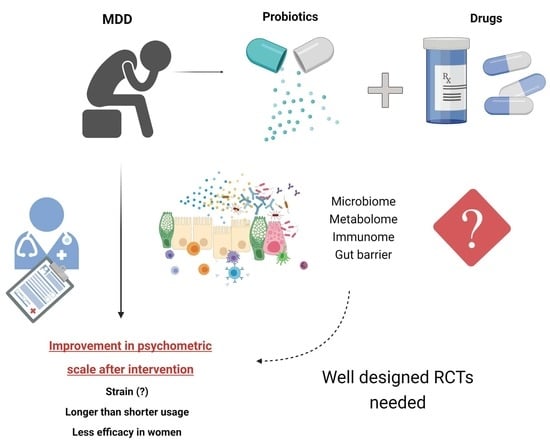
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Inclusion Criteria
- clinical diagnosis of MDD,
- probiotic or psychobiotic or symbiotic administration,
- RCT design,
- meta-analyzable endpoint/change score data on any of the psychometric tests.
4.2. Data Abstraction
4.3. Outcomes
4.4. Data Synthesis and Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 8 August 2021).
- Berlim, M.T.; Turecki, G. What Is the Meaning of Treatment Resistant/Refractory Major Depression (TRD)? A Systematic Review of Current Randomized Trials. Eur. Neuropsychopharmacol. 2007, 17, 696–707. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.F.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W.J.H. Association of Depressive Disorders, Depression Characteristics and Antidepressant Medication with Inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef] [Green Version]
- Vreeburg, S.A.; Hoogendijk, W.J.G.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.M.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W.J.H. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity: Results from a Large Cohort Study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molendijk, M.L.; Bus, B.A.; Spinhoven, P.; Penninx, B.W.J.H.; Kenis, G.; Prickaerts, J.; Voshaar, R.C.O.; Elzinga, B.M. Serum Levels of Brain-Derived Neurotrophic Factor in Major Depressive Disorder: State-Trait Issues, Clinical Features and Pharmacological Treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota−Gut−Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut Microbiota and Major Depressive Disorder: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Flux, M.C.; Lowry, C.A. Finding Intestinal Fortitude: Integrating the Microbiome into a Holistic View of Depression Mechanisms, Treatment, and Resilience. Neurobiol. Dis. 2020, 135, 104578. [Google Scholar] [CrossRef]
- Łoniewski, I.; Misera, A.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Kaźmierczak-Siedlecka, K.; Misiak, B.; Marlicz, W.; Samochowiec, J. Major Depressive Disorder and Gut Microbiota-Association Not Causation. A Scoping Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110111. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and Probiotics for Depression and Anxiety: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.K.; Liu, Y.-W.; Kuo, P.-H.; Chung, Y.-C.E.; Lu, M.-L.; Chen, C.-H. Effect of Probiotics on Depressive Symptoms: A Meta-Analysis of Human Studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef]
- Nikolova, V.; Zaidi, S.Y.; Young, A.H.; Cleare, A.J.; Stone, J.M. Gut Feeling: Randomized Controlled Trials of Probiotics for the Treatment of Clinical Depression: Systematic Review and Meta-Analysis. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319859963. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. J. Clin. Med. 2021, 10, 647. [Google Scholar] [CrossRef]
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From Probiotics to Psychobiotics-the Gut-Brain Axis in Psychiatric Disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The Effects of Probiotic Supplementation on Mental Health, Biomarkers of Inflammation and Oxidative Stress in Patients with Psychiatric Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A Meta-Analysis of the Use of Probiotics to Alleviate Depressive Symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of Probiotic and Prebiotic vs Placebo on Psychological Outcomes in Patients with Major Depressive Disorder: A Randomized Clinical Trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of Prebiotic and Probiotic Supplementation on Circulating Pro-Inflammatory Cytokines and Urinary Cortisol Levels in Patients with Major Depressive Disorder: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Jahromi, S.R. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef] [Green Version]
- Heidarzadeh-Rad, N.; Gokmen-Ozel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-Derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Reininghaus, E.Z.; Platzer, M.; Kohlhammer-Dohr, A.; Hamm, C.; Mörkl, S.; Bengesser, S.A.; Fellendorf, F.T.; Lahousen-Luxenberger, T.; Leitner-Afschar, B.; Schöggl, H.; et al. PROVIT: Supplementary Probiotic Treatment and Vitamin B7 in Depression—A Randomized Controlled Trial. Nutrients 2020, 12, 3422. [Google Scholar] [CrossRef]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.-C.; Birkl-Töglhofer, A.-M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.-M.; Unterweger, R.; et al. Interleukin-6 Gene Expression Changes after a 4-Week Intake of a Multispecies Probiotic in Major Depressive Disorder-Preliminary Results of the PROVIT Study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020, 22, 19m02578. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut Feelings: A Randomised, Triple-Blind, Placebo-Controlled Trial of Probiotics for Depressive Symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.-D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.-S.; et al. The Effect of a Multispecies Probiotic Mixture on the Symptoms and Fecal Microbiota in Diarrhea-Dominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.-M.; Botelho, C.; et al. Functional Dynamics of the Gut Microbiome in Elderly People during Probiotic Consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormel, G.; Hemert, S.V. Influence of the Multispecies Probiotic Ecologic® BARRIER on Parameters of Intestinal Barrier Function. Food Nutr. Sci. 2014, 5, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-National Epidemiology of DSM-IV Major Depressive Episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, M.J.; Hong, J.P.; Bae, J.N.; Cho, S.-J.; Hahm, B.-J.; Lee, D.-W.; Park, J.-I.; Lee, J.-Y.; Jeon, H.J.; et al. Gender Differences in Depressive Symptom Profile: Results from Nationwide General Population Surveys in Korea. J. Korean Med. Sci. 2015, 30, 1659–1666. [Google Scholar] [CrossRef] [Green Version]
- Kornstein, S.G.; Schatzberg, A.F.; Thase, M.E.; Yonkers, K.A.; McCullough, J.P.; Keitner, G.I.; Gelenberg, A.J.; Ryan, C.E.; Hess, A.L.; Harrison, W.; et al. Gender Differences in Chronic Major and Double Depression. J. Affect. Disord. 2000, 60, 1–11. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zheng, P.; Liu, Y.-Y.; Zhong, X.-G.; Wang, H.-Y.; Guo, Y.-J.; Xie, P. Sex Differences in Gut Microbiota in Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the Mammalian Brain: When Physiology Meets Pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S.; et al. Exploration of Microbiota Targets for Major Depressive Disorder and Mood Related Traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-T.; Deng, W.-F.; Xu, S.-X.; Zhao, J.; Xu, D.; Liu, Y.-H.; Guo, Y.-Y.; Wang, M.-B.; He, F.-S.; Ye, S.-W.; et al. Shotgun Metagenomics Reveals Both Taxonomic and Tryptophan Pathway Differences of Gut Microbiota in Major Depressive Disorder Patients. Psychol. Med. 2021, 51, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A Double-Blind, Randomized, Placebo-Controlled Trial of Lactobacillus Helveticus and Bifidobacterium Longum for the Symptoms of Depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- PRISMA. Available online: http://www.prisma-statement.org/Protocols/ (accessed on 8 August 2021).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]








| Study Description | Intervention | Sample Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference/Country/Sponsorship/Registration No. | Blinding/ Setting | Diagnostic Criteria | Treatment | N total Randomized/ Analyzed | Number of Patients Per Study Group | Probiotic Strain | Duration (Days) | Probiotic Daily Dose (CFU) | Comparator | Age (Mean ± SD) | Females (n, %) | BMI (Mean ± SD) |
| Rudzki et al., 2019 [28]/Poland/Academia/ ClinicalTrials.gov: NCT02469545 (access date 20 August 2021) | DB/ Outpatient | DSM IV-R | Current SSRI monotherapy (n = 9), beginning of SSRIs treatment (n = 21) | 79/60 | S: n = 30, C: n = 30 | L. plantarum 299v | 56 | 2 × 1010 | Placebo | Whole group: 39.02 ± 11.0; S: 39.13 ± 9.96; C: 38.9 ± 12; | S: n = 23 (76.7); C: n = 20 (66.7) | Whole group: 23.82 ± 3.47; S: 24.09 ± 3.76; C: 23.55 ± 3.13 |
| $ Kazemi et al., 2019a,b [21,22]; /Iran/nd/ www.irct.ir: IRCT2015092924271N1 (access date 20 August 2021) | DB/ Outpatient | ND | Sertraline, fluoxetine, citalopram or amitriptyline ≥3 months before the trial. | 74/74 for BDI; 54 other outcomes | S: n = 38 for BDI; 28 other outcomes, C: n = 36 for BDI; 26 other outcomes | L. helveticus R0052 (CNCM strain I-1722) and B. longum R0175 (CNCM strain I-3470) | 2 months | 1 × 1010 | Placebo | Whole group (ITT): 36.07 ± 5.84; S:36.15 ± 7.85; C: 36 ± 8.47 | S (ITT): n = 27 (71); C (ITT): N = 24 (66.6) | Whole group (ITT): 26.35 ± 4.57; S (ITT): 26.11 ± 4; C (ITT): 26.61 ± 4.97 |
| $ Heidarzadeh-Rad et al., 2020 [24] /Iran/academia/ www.irct.ir: IRCT2015092924271N1 (access date 20 August 2021) | DB/ Outpatient | ND | Sertraline, fluoxetine, citalopram or amitriptyline ≥3 months before the trial. | 74/53 ^ | S: n = 28, C: n = 25 | L. helveticus R0052 (CNCM strain I-1722) and B. longum R0175 (CNCM strain I-3470) | 2 months | 1 × 1010 | Placebo | Whole group: 36.95 ± 8.24; S: 37.8 ± 7.9/ C: 36.0 ± 8.5 | S: n = 20 (71.4); C: n = 15 (60) | Whole group: 26.69 ± 4.35; S: 26.6 ± 4.2; C: 26.8 ± 4.5 |
| Ghorbani et al., 2018 [23] /Iran/academia/ Shahid Behesti University of Medical Science ethics committee (code: 1394/s/35029) | DB/ Outpatient | DSM-V | Fluoxetine (20 mg/d) for 4 weeks before study | 40/40 | S: n = 20, C: n = 20 | L. casaei, L. acidofilus, L. bulgarigus, L. rhamnosus, B. breve, B. longum, S. thermophilu and fructooligosaccharide | 42 | L. casaei 3 × 108; L. acidofilus 2 × 108, L. bulgarigus 2 × 109, L. rhamnosus 3 × 108, B. breve 2 × 108, B. longum 1 × 109, S. thermophilus 3 × 108 and 200 mg fructooligosaccharide | Placebo | Whole group: 34.97 ± 4.68; S: 34.45 ± 3.95; C: 35.5 ± 5.27 | S: n = 14 (70); C: n = 14 (70) | Whole group: 24.07 ± 5.03; S: 24.04 ± 5.25; C: 24.11 ± 4.81 |
| Chahwan et al., 2019 [30]/ Australia and Netherlands/academia/ ANZCTR, Trial ID: ACTRN12615001081505 | TB/ Outpatient | BDI-II | None | 71/71 | S: n = 34, C: n = 37; Non-depressed control: n = 20 (microbiome analysis) | B. bifidum W23, B. lactis W51 and W52, L. acidophilus W37, L. brevis W63, L.casei W56, L. salivarius W24, L. lactis W19 W58 | 56 | 1 × 1010 | Placebo | Whole group (S + C): 36.04 ± 12.07; S: 36.65 ± 11.75/ C: 35.49 ± 12.34 | S: n = 21 (61.76); C: n = 28 (75.67); | ND |
| Akkasheh et al., 2015 [25]/Iran/nd/ www.irct.ir: IRCT2014060717993N1 (access date 20 August 2021) | DB/ Outpatient | DSM-IV; ≥15 in HAM-D 17-item | ND | 40/40 | S: n = 20, C: n = 20 | L. acidophilus, L. casei and B. bifidum | 56 | 1 capsule daily, content of each—bacteria 2 × 109 CFU/g, weight of capsules was not given. | Placebo | Whole group: 37.25 ± 10.38; S: 38.3 ± 12.1/ C: 36.2 ± 8.2 | ND | Whole group: 26.95 ± 5.18; S: 27.6 ± 6.0; C: 26.3 ± 4.1 |
| Saccarello et al., 2020 [29]/Italy/industry/ ClinicalTrials.gov: NCT03932474 (access date 20 August 2021) | DB/ Outpatient | ICD-10 (F33.0); ZSDS score 41–55 | None | 90/87 | S: n = 43, C: n = 44 | Lactobacillus plantarum Heal 9 + SAMe | 42 | 1 × 109 + 200 mg SAMe | Placebo | Whole group: 48.1 ± 11.25; S: 48.6 ± 10.67/C: 47.5 ± 11.9 | S: n = 38 (84.4); C: n = 35 (79.5) | Whole group: 24.3 ± 5.44; S: 24.1 ± 6.16; C: 24.5 ± 4.64 |
| Reininghaus et al., 2020 * [26]/ Reiter et al., 2020 * [27]/ Austria/academia/ ClinicalTrials.gov: NCT03300440 (access date 20 August 2021) | DB/ Inpatient | M.I.N.I. | 125 mg D-Biotin (vitamin B7), 30 mg of common horsetail, 30 mg of fish collagen and 30 mg of keratin plus matrix. Psychiatric treatment: ## | 82/61 | S: n = 28, C: n = 33 | B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W22, L. casei W56, L. paracasei W20, L. plantarum W62, L. salivarius W24, L. lactis W19 and FOS | 28 | 7.5 × 109 | Placebo | Whole group: 41.44 ± 12.92; S: 43.00 ± 14.31; C: 40.11 ± 11.45 | S: n = 20 (71.4); C: n = 27 (81.8) | Whole group: 25.99 ± 6.65; S: 26.29 ± 5.78; C: 25.74 ± 7.29 |
| Reference/Country/Sponsorship | Major Study Focus/Outcome | Discontinuation: All Cause | Discontinuation: Adverse Effects | Adverse Effects (%) | |||
|---|---|---|---|---|---|---|---|
| PRO | PBO | PRO | PBO | PRO | PRO | ||
| Rudzki et al., 2019 [28]/Poland/Academia | Influence of psychobiotic administration on cognitive, affective and immune parameters of Major Depressive Disorder (MDD) patients treated with SSRIs /improvement of cognitive performance and decrease of blood kynurenine concentration after psychobiotic treatment. | 10/40 | 9/39 | 0 | 0 | Headache (13.3), Loose Stool (3.3), Flatulence (3.3), Palpitations (3.3). | Headache (6.6), Vertigo (3.3), Tremor (3.3), Loose Stool (3.3). |
| $ Kazemi et al., 2019a [21]/Iran/nd | Influence of psychobiotic administration on Beck Depression Inventory (BDI) score; kynurenine, tryptophan and Branch chain amino acids (BCAAs) blood concentration in MDD patients treated with antidepressive agents/improvement in BDI score, decrease in the kynurenine:tryptophan ratio adjusted for serum isoleucine. | 10/36 | 10/38 | 5 | 0 | Gi complaints (5.56), Nausea (2.78), Fever and body aches (2.78), Increased appetite (13.89). | 0 |
| $ Kazemi et al., 2019b [22]/Iran/nd | Effect of psychobiotic treatment on blood pro-inflammatory cytokines and the urinary cortisol level in MDD patients treated with antidepressive agents/clinically (not statistically) significant change of urine cortisol concentration. | ||||||
| $ Heidarzadeh-Rad et al., 2020 [24]/Iran/academia | Effect of psychobiotics on serum BDNF in MDD patients treated with antidepressive agents/increase in BDNF, which was inversely correlated with depression severity. | 11 ^/36 | 10/38 | 5 | 0 | Gi complaints (5.56), Nausea (2.78), Fever and body aches (2.78), increased appetite (13.89). | 0 |
| Ghorbani et al., 2018 [23]/Iran/academia | Influence of synbiotic on HAM-D 17-item score in MDD patients treated with fluoxetine/significant decrease of HAM-D 17-item score. | 0/20 | 0/20 | 0/20 | 0/20 | Bloating (20), Diarrhea (10), Abdominal Cramps (15), Nausea (20). | Bloating (5), Nausea (10). |
| Chahwan et al., 2019 [30]/Australia and Netherlands/academia | The primary aim: influence of probiotic on the reduction in depressive symptoms. A secondary aims: 1. the treatment response depending on baseline levels of depression; 2. effects of the probiotics on cognitive reactivity, 3. gut microbiota analysis/greater reduction in cognitive reactivity in probiotic group. | 11/34 | 13/37 | 0/34 | 0/37 | Nausea (32.35), Abdominal/Stomach Pain/Discomfort (26.47), Dehydration (26.47), Drowsiness (20.59) #, Bloating (14.71), Flatulence (11.76), Change in bowels (11.76), Dizzy (8.82), Constipation (8.82), Diarrhoea (5.88), Rash/Itchy (5.88), Vomiting (5.88), Unpleasant Taste (5.88), Headache (5.88). | Nausea (16.22), Abdominal/Stomach Pain/Discomfort (18.92), Dehydration (24.32), Drowsiness (2.7), Bloating (2.7), Flatulence (5.41), Change in bowels (2.70), Dizzy (10.81), Diarrhoea (8.11), Rash/Itchy (5.41), Vomiting (2.7), Unpleasant taste (2.7), Dry Mouth (13.51). |
| Akkasheh et al., 2015 [25]/Iran/nd | Effects of probiotics on symptoms of depression and metabolic parameters/ favorable effect on BDI and decrease serum insulin, HOMA-IR, serum hs-CRP; increase GSH. | 3/20 | 2/20 | ND | ND | ND | |
| Saccarello et al., 2020 [29]/Italy/industry | Primary: influence of symbiotic on overall symptomatology of depression. Secondary: 1. effects of treatment on symptoms associated with depression, 2. overall health status and 3. safety assessment/significantly improved symptoms of depression, anxiety and cognitive and somatic comoponents. | 2/45 | 0/44 | 0/45 | 0/44 | Reduced appetite and low mood (4.44) | Rash, rrythema and itching (2.22); |
| * Reininghaus et al., 2020 [26]/Austria/academia | Influence of treatment on overall symptomatology of depression and gut microbiota/No significant effect of probiotics on clinical symptoms. Observed effect on gut microbiota. | 14/42 | 7/40 | 0/42 | 0/40 | ND | |
| * Reiter et al., 2020 [27]/Austria/academia | Influence of probiotics on the expression of inflammation-related genes/Decreased expression of IL-6. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misera, A.; Liśkiewicz, P.; Łoniewski, I.; Skonieczna-Żydecka, K.; Samochowiec, J. Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression. Pharmaceuticals 2021, 14, 952. https://doi.org/10.3390/ph14100952
Misera A, Liśkiewicz P, Łoniewski I, Skonieczna-Żydecka K, Samochowiec J. Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression. Pharmaceuticals. 2021; 14(10):952. https://doi.org/10.3390/ph14100952
Chicago/Turabian StyleMisera, Agata, Paweł Liśkiewicz, Igor Łoniewski, Karolina Skonieczna-Żydecka, and Jerzy Samochowiec. 2021. "Effect of Psychobiotics on Psychometric Tests and Inflammatory Markers in Major Depressive Disorder: Meta-Analysis of Randomized Controlled Trials with Meta-Regression" Pharmaceuticals 14, no. 10: 952. https://doi.org/10.3390/ph14100952