Inhalation of Essential Oil from Mentha piperita Ameliorates PM10-Exposed Asthma by Targeting IL-6/JAK2/STAT3 Pathway Based on a Network Pharmacological Analysis
Abstract
:1. Introduction
2. Results
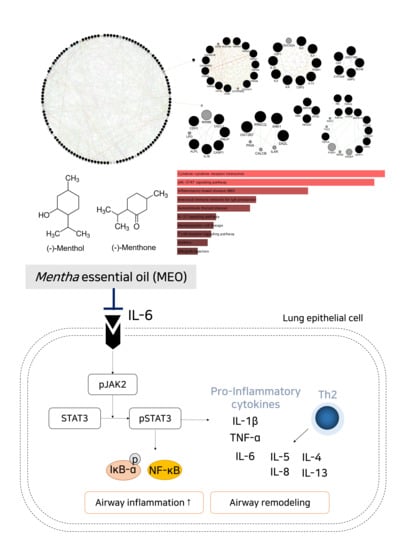
2.1. Construction of Menthol and Menthone Network
2.2. Module Selection and Cluster of the Enriched Pathway Analysis Using Predicted Targets of Menthol and Menthone
2.3. Effects of Aerosolized MEO on Histological Changes in PM10-Exposed Lung Tissues of Mice
2.4. Effects of MEO on Inflammatory Cytokines Levels in PM10-Sensitized A549 Cells
2.5. Effects of MEO on Proliferative MMPs, Especially MMP-2 and MMP-9, Levels in PM10-Sensitized A549 Cells
2.6. Effects of MEO on JAK/STAT3 Signaling Pathway in PM10-Sensitized A549 Cells
2.7. Effects of MEO on NF-κB Translocation into Nucleus in PM10-Sensitized A549 Cells
3. Discussion
4. Materials and Methods
4.1. Network Construction
4.2. Pathway Analysis and Module Analysis
4.3. Peppermint Oil Extraction from Mentha Piperita Linn
4.4. PM10-Exposed Animal Treatment
4.5. Histology
4.6. PM10-Exposed in Vitro Experiments
4.7. Western Blot Analysis
4.8. RNA Extraction and RT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quirt, J.; Hildebrand, K.J.; Mazza, J.; Noya, F.; Kim, H. Asthma. Allergy Asthma Clin. Immunol. 2018, 14, 50. [Google Scholar] [CrossRef]
- Jo, E.-J.; Lee, W.-S.; Jo, H.-Y.; Kim, C.-H.; Eom, J.-S.; Mok, J.-H.; Kim, M.-H.; Lee, K.; Kim, K.-U.; Lee, M.-K.; et al. Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respir. Med. 2017, 124, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Michael Guarnieri, J.R.B. Outdoor air pollution and asthma. Food Chem. Toxicol. 1996, 34, 318–319. [Google Scholar]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Irvin, C.G. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Cho, M.; Choi, G.; Na, H.; Chung, Y. Dynamic control of Th2 cell responses by STAT3 during allergic lung inflammation in mice. Int. Immunopharmacol. 2015, 28, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Tal, T.; Graves, L.M.; Gilmour, I.; Linak, W.; Reed, W.; Bromberg, P.A.; Samet, J.M. Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L422–L429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhang, Z.; Ma, X.; Ping, F.; Zheng, X. [Effect of PM2.5 on oxidative stress-JAK/STAT signaling pathway of human bronchial epithelial cells]. Wei Sheng Yan Jiu 2015, 44, 451–455. [Google Scholar] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Mohd-Setapar, S.H.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.-H.; Rehman, N. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytotherapy Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of Essential Oils of the Genus Mentha as Biopesticides: A Review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rasal, V.P.; Patil, P.A.; Joshi, R.K. Mentha arvensis essential oil suppressed airway changes induced by histamine and ovalbumin in experimental animals. Nat. Prod. Res. 2018, 32, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Al Zabadi, H.; Rahhal, B.; Hussein, A.M.A.; Mahmoud, J.S.; Mansour, B.; Khasati, A.I.; Issa, A. The effect of inhalation of Citrus sinensis flowers and Mentha spicata leave essential oils on lung function and exercise performance: A quasi-experimental uncontrolled before-and-after study. J. Int. Soc. Sports Nutr. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K.E. Antifungal properties of essential oils for improvement of indoor air quality: A review. Rev. Environ. Heal. 2018, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.; Finch, J.; Edwards, K.; Jeanjean, A.; Leigh, R.; Gonem, S. Effects of personal air pollution exposure on asthma symptoms, lung function and airway inflammation. Clin. Exp. Allergy 2018, 48, 798–805. [Google Scholar] [CrossRef] [Green Version]
- Camelo, A.; Dunmore, R.; Sleeman, M.A.; Clarke, D.L. The epithelium in idiopathic pulmonary fibrosis: Breaking the barrier. Front. Pharmacol. 2014, 4, 173. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ning, W.; Xu, Y.; Hua, L.; Zhou, D.; Zheng, M.; Deng, X. Effects of chronic PM2.5 exposure on pulmonary epithelia: Transcriptome analysis of mRNA-exosomal miRNA interactions. Toxicol. Lett. 2019, 316, 49–59. [Google Scholar] [CrossRef]
- Kumar, R.K.; Shadie, A.M.; Bucknall, M.; Rutlidge, H.; Garthwaite, L.; Herbert, C.; Halliburton, B.; Parsons, K.; Wark, P.A. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology 2015, 20, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Maglione, G.A.; Kurtz, M.L.; Orona, N.S.; Astort, F.; Busso, I.T.; Mandalunis, P.M.; Berra, A.; Tasat, D.R. Chronic exposure to urban air pollution from Buenos Aires: The ocular mucosa as an early biomarker. Environ. Sci. Pollut. Res. 2019, 26, 27444–27456. [Google Scholar] [CrossRef]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Wu, J.; Li, Q.; Asweto, C.; Feng, L.; Yang, X.; Duan, F.; Duan, J.; Sun, Z. Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicol. Res. 2016, 5, 946–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Taş, I.; Zhou, R.; Park, S.-Y.; Yang, Y.; Gamage, C.D.; Son, Y.-J.; Paik, M.-J.; Kim, H. Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicol. In Vitro 2019, 59, 300–311. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, N.; Wang, Z.; Wu, C.; Sun, Z.; Li, Y.; Hu, F.; Wang, Z.; Huang, M.; Zhang, M. Fine Particulate Matter (PM2.5) Promoted the Invasion of Lung Cancer Cells via an ARNT2/PP2A/STAT3/MMP2 Pathway. J. Biomed. Nanotechnol. 2018, 14, 2172–2184. [Google Scholar] [CrossRef]
- Reyes-Zárate, E.; Sánchez-Pérez, Y.; Gutiérrez-Ruiz, M.; Chirino, Y.I.; Osornio-Vargas, A.R.; Morales-Bárcenas, R.; Souza-Arroyo, V.; García-Cuellar, C.M. Atmospheric particulate matter (PM10) exposure-induced cell cycle arrest and apoptosis evasion through STAT3 activation via PKCζ and Src kinases in lung cells. Environ. Pollut. 2016, 214, 646–656. [Google Scholar] [CrossRef]
- Yeh, T.C.; Pellegrini, S. The Janus kinase family of protein tyrosine kinases and their role in signaling. Cell. Mol. Life Sci. 1999, 55, 1523–1534. [Google Scholar] [CrossRef]
- Wegmann, M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert Rev. Mol. Diagn. 2009, 9, 85–100. [Google Scholar] [CrossRef]
- Simeone-Penney, M.C.; Severgnini, M.; Tu, P.; Homer, R.J.; Mariani, T.J.; Cohn, L.; Simon, A.R. Airway Epithelial STAT3 Is Required for Allergic Inflammation in a Murine Model of Asthma. J. Immunol. 2007, 178, 6191–6199. [Google Scholar] [CrossRef]
- Mazzarella, G.; Esposito, V.; Bianco, A.; Ferraraccio, F.; Prati, M.; Lucariello, A.; Manente, L.; Mezzogiorno, A.; De Luca, A. Inflammatory effects on human lung epithelial cells after exposure to diesel exhaust micron sub particles (PM1.0) and pollen allergens. Environ. Pollut. 2012, 161, 64–69. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lewis, D.F.; Gu, Y.; Zhao, S.; Groome, L.J. Elevated Maternal Soluble Gp130 and IL-6 Levels and Reduced Gp130 and SOCS-3 Expressions in Women Complicated With Preeclampsia. Hypertension 2011, 57, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagouassat, M.; Lanone, S.; Boczkowski, J. Interaction of matrix metalloproteinases with pulmonary pollutants. Eur. Respir. J. 2012, 39, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Karin, M. NF-κB and STAT3–key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Herrmann, A.; Deng, J.-H.; Kujawski, M.; Niu, G.; Li, Z.; Forman, S.; Jove, R.; Pardoll, D.M.; Yu, H. Persistently Activated Stat3 Maintains Constitutive NF-κB Activity in Tumors. Cancer Cell 2009, 15, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of Essential Oil from Mentha x piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, S.; Ray, D.; Warde-Farley, D.; Grouios, C.; Morris, Q.D. GeneMANIA: A real-time multiple association network integration algorithm for predicting gene function. Genome Biol. 2008, 9 (Suppl. 1), S4. [Google Scholar] [CrossRef] [Green Version]
- Assenov, Y.; Ramírez, F.; Schelhorn, S.-E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [Green Version]
- Denkers, N.D.; Seelig, D.M.; Telling, G.C.; Hoover, E.A. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J. Gen. Virol. 2010, 91, 1651–1658. [Google Scholar] [CrossRef]







| Module | KEGG Pathway 2019 Human | p Value | GO Biological Process 2018 | p Value |
|---|---|---|---|---|
| M1 | Mineral absorption | 6.5 × 10−4 | calcium ion transport (GO:0006816) | 2.5 × 10−22 |
| M2 | Cytokine–cytokine receptor interaction | 2.8 × 10−16 | cellular response to cytokine stimulus (GO:0071345) | 1.5 × 10−14 |
| M3 | Retinol metabolism | 1.1 × 10−4 | coumarin metabolic process (GO:0009804) | 1.5 × 10−3 |
| M4 | Other glycan degradation | 3.6 × 10−3 | positive regulation of action potential (GO:0045760) | 1.2 × 10−3 |
| M5 | Legionellosis | 1.1 × 10−6 | response to organic cyclic compound (GO:0014070) | 3.2 × 10−4 |
| M6 | Th1 and Th2 cell differentiation | 4.3 × 10−4 | positive regulation of T-helper 17 type immune response (GO:2000318) | 2.1 × 10−3 |
| M7 | Neuroactive ligand-receptor interaction | 9.4 × 10−5 | positive regulation of cytosolic calcium ion concentration (GO:0007204) | 2.4 × 10−6 |
| Cluster No. | KEGG Pathway 2019 Human | p Value |
|---|---|---|
| Cluster 1 | Cytokine–cytokine receptor interaction | 2.779 × 10−16 |
| Cluster 2 | JAK-STAT signaling pathway | 6.906 × 10−16 |
| Cluster 3 | Inflammatory bowel disease (IBD) | 1.932 × 10−13 |
| Cluster 4 | Intestinal immune network for IgA production | 1.604 × 10−11 |
| Cluster 5 | Autoimmune thyroid disease | 2.685 × 10−11 |
| Cluster 6 | IL-17 signaling pathway | 4.823 × 10−10 |
| Cluster 7 | Hematopoietic cell lineage | 5.976 × 10−10 |
| Cluster 8 | T cell receptor signaling pathway | 7.339 × 10−10 |
| Cluster 9 | Asthma | 9.848 × 10−10 |
| Cluster 10 | Allograft rejection | 2.307 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Park, S.J.; Yang, W.M. Inhalation of Essential Oil from Mentha piperita Ameliorates PM10-Exposed Asthma by Targeting IL-6/JAK2/STAT3 Pathway Based on a Network Pharmacological Analysis. Pharmaceuticals 2021, 14, 2. https://doi.org/10.3390/ph14010002
Kim MH, Park SJ, Yang WM. Inhalation of Essential Oil from Mentha piperita Ameliorates PM10-Exposed Asthma by Targeting IL-6/JAK2/STAT3 Pathway Based on a Network Pharmacological Analysis. Pharmaceuticals. 2021; 14(1):2. https://doi.org/10.3390/ph14010002
Chicago/Turabian StyleKim, Mi Hye, Sang Jun Park, and Woong Mo Yang. 2021. "Inhalation of Essential Oil from Mentha piperita Ameliorates PM10-Exposed Asthma by Targeting IL-6/JAK2/STAT3 Pathway Based on a Network Pharmacological Analysis" Pharmaceuticals 14, no. 1: 2. https://doi.org/10.3390/ph14010002