Microwave-Assisted Synthesis of Potential Bioactive Benzo-, Pyrido- or Pyrazino-thieno[3,2-d]pyrimidin-4-amine Analogs of MPC-6827
Abstract
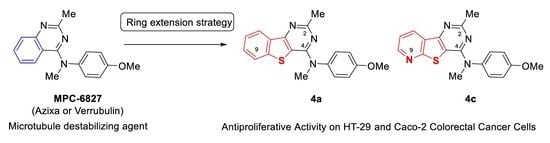
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiproliferative Activity on Colorectal Cancer Cell Lines (Caco-2 and HT-29)
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. General Information
4.1.2. General Procedure for the Synthesis of N,N-dimethylacetimidamide Derivatives (2a–d)
4.1.3. General Procedure for the Synthesis of N-(4-methoxyphenyl)-2-methylthieno[3,2-d]pyrimidin-4-amines (3a–d)
4.1.4. General Procedure for the Synthesis of N-(4-methoxyphenyl)-N,2-dimethylthieno[3,2-d]pyrimidin-4-amines (4a–d)
4.2. Antiproliferative Activity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Raja Solomon, V.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Abid Ali, S.; Marium, A.; Khalid, I.; Khan, M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; Zhang, Q.; Yu, W.; Liu, M.; Guo, Y.; He, J.; Liu, Y. The association between anti-tumor potency and structure-activity of protein-kinases inhibitors based on quinazoline molecular skeleton. Bioorg. Med. Chem. 2019, 27, 568–577. [Google Scholar] [CrossRef]
- Das, D.; Hong, J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019, 170, 55–72. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Baichwal, V.; Cai, S.X.; Roth, B.; Skvortsova, I.; Skvortsov, S.; Lukas, P.; English, N.M.; Sirisoma, N.; Drewe, J.; et al. MPC-6827: A small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007, 67, 5865–5871. [Google Scholar] [CrossRef] [Green Version]
- Sirisoma, N.; Kasibhatla, S.; Pervin, A.; Zhang, H.; Jiang, S.; Willardsen, J.A.; Anderson, M.B.; Baichwal, V.; Mather, G.G.; KJessing, K.; et al. Discovery of 2-chloro-N-(4-methoxyphenyl)-N-methylquinazolin-4-amine (EP128265, MPI-0441138) as a potent inducer of apoptosis with high in vivo activity. J. Med. Chem. 2008, 51, 4771–4779. [Google Scholar] [CrossRef]
- Kemnitzer, W.; Sirisoma, N.; May, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery of 4-anilino-N-methylthieno[3,2-d]pyrimidines and 4-anilino-N-methylthieno[2,3-d]pyrimidines as potent apoptosis inducers. Bioorg. Med. Chem. Lett. 2009, 19, 3536–3540. [Google Scholar] [CrossRef]
- Sirisoma, N.; Pervin, A.; Zhang, H.; Jiang, S.; Willardsen, J.A.; Anderson, M.B.; Mather, G.G.; Jessing, K.; Pleiman, C.M.; Kasibhatla, S.; et al. Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration. J. Med. Chem. 2009, 52, 2341–2351. [Google Scholar] [CrossRef]
- Sirisoma, N.; Pervin, A.; Zhang, H.; Jiang, S.; Adam Willardsen, J.; Anderson, M.B.; Mather, G.; Pleiman, C.M.; Kasibhatla, S.; Tseng, B.; et al. Discovery of N-methyl-4-(4-methoxyanilino)quinazolines as potent apoptosis inducers. Structure-activity relationship of the quinazoline ring. Bioorg. Med. Chem. Lett. 2010, 20, 2330–2334. [Google Scholar] [CrossRef]
- Mahal, K.; Resch, M.; Ficner, R.; Schobert, R.; Biersack, B.; Mueller, T. Effects of the tumor-vasculature-disrupting agent Verubulin and two heteroaryl analogues on cancer cells, endothelial cells, and blood vessels. ChemMedChem 2014, 9, 847–854. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Akerley, W.; Schabel, M.C.; Hong, D.S.; Uehara, C.; Chhabra, A.; Warren, T.; Mather, G.G.; Evans, B.A.; Woodland, D.P.; et al. Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol. Cancer Ther. 2010, 9, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K.F.; Colman, H.; Akerley, W.A.; Glantz, M.; Matsuoko, Y.; Beelen, A.P.; Yu, M.; De Groot, J.F.; Aiken, R.D.; Olsen, J.J.; et al. Phase I trial of verubulin (MPC-6827) plus carboplatin in patients with relapsed glioblastoma multiform. J. Neurooncol. 2012, 110, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Grimm, S.; Phuphanich, S.; Recht, L.; Zhu, J.Z.; Kim, L.; Rosenfeld, S.; Fadul, C.E. A phase 2 trial of verubulin for recurrent glioblastoma: A prospective study by the brain tumor investigational consortium (BTIC). J. Neurooncol. 2014, 118, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Subbiaha, I.M.; Lenihanb, D.J.; Tsimberidouc, A.M. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist 2011, 16, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yin, Y.; Shuai, W.; Xu, F.; Yao, H.; Liu, J.; Cheng, K.; Xu, J.; Zhu, Z.; Xu, S. Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain. Bioorg. Chem. 2019, 83, 380–390. [Google Scholar] [CrossRef]
- Loidreau, Y.; Besson, T. Microwave-assisted thermal decomposition of formamide: A tool for coupling a pyrimidine ring with an aromatic partner. Tetrahedron 2011, 67, 4852–4857. [Google Scholar] [CrossRef]
- Hédou, D.; Deau, E.; Dubouilh-Benard, C.; Sanselme, M.; Martinet, A.; Chosson, E.; Levacher, V.; Besson, T. Microwave-assisted (3 + 2) cycloaddition and Suzuki-Miyaura cross-coupling for a concise access to novel polyaromatic scaffolds. Eur. J. Org. Chem. 2013, 7533–7545. [Google Scholar] [CrossRef]
- Deau, E.; Hédou, D.; Chosson, E.; Levacher, V.; Besson, T. Convenient one-pot synthesis of N3-substituted pyrido[2,3-d]-, pyrido[3,4-d]-, pyrido[4,3-d]-pyrimidin-4(3H)-ones, and quinazolin-4(3H)-ones analogs. Tetrahedron Lett. 2013, 54, 3518–3521. [Google Scholar] [CrossRef]
- Foucourt, A.; Dubouilh-Benard, C.; Chosson, E.; Corbière, C.; Buquet, C.; Iannelli, M.; Leblond, B.; Marsais, F.; Besson, T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 2010, 66, 4495–4502. [Google Scholar] [CrossRef]
- Loidreau, Y.; Dubouilh-Benard, C.; Marchand, P.; Nourrisson, M.-R.; Duflos, M.; Buquet, C.; Corbière, C.; Besson, T. Efficient new synthesis of N-arylbenzofuro[3,2-d]pyrimidin-4-amines and their novel benzo[b]thieno[3,2-d]pyrimidin-4-amine analogues via a microwave-assisted Dimroth rearrangement. J. Heterocycl. Chem. 2013, 50, 1187–1197. [Google Scholar] [CrossRef]
- Brocklesby, K.L.; Waby, J.S.; Cawthorne, C.; Smith, G. An alternative synthesis of Vandetanib (CaprelsaTM) via a microwave accelerated Dimroth rearrangement. Tetrahedron Lett. 2017, 58, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Marinho, E.; Proença, M.F. The Reaction of 2-(acylamino)benzonitriles with primary aromatic amines: A convenient synthesis of 2-substituted 4-(arylamino)quinazolines. Synthesis 2015, 47, 1623–1632. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, L.; Leleu-Chavain, N.; Tourteau, A.; Abdul-Sada, A.; Spencer, J.; Millet, R. A rapid route for the preparation of pyrimido[5,4-d]- and pyrido[3,2-d]oxazoles. Tetrahedron Lett. 2015, 56, 2448–2450. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Mirallai, S.I. Reinvestigating the synthesis of N-arylbenzamidines from benzonitriles and anilines in the presence of AlCl3. Tetrahedron 2010, 66, 5134–5139. [Google Scholar] [CrossRef]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Lozach, O.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012, 58, 171–183. [Google Scholar] [CrossRef]
- Loidreau, Y.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Loaëc, N.; Meijer, L.; Besson, T.; Marchand, P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals 2020, 13, 89. [Google Scholar] [CrossRef]
- Loidreau, Y.; Melissen, S.; Levacher, V.; Logé, C.; Graton, J.; Le Questel, J.Y.; Besson, T. Study of N1-alkylation of indoles from the reaction of 2(or 3)-aminoindole-3-(or 2)carbonitriles with DMF-dialkylacetals. Org. Biomol. Chem. 2012, 20, 4916–4925. [Google Scholar] [CrossRef]
- Lin-Lee, Y.C.; Tatebe, S.; Savaraj, N.; Ishikawa, T.; Tien Kuo, M. Differential sensitivities of the MRP gene family and γ-glutamylcysteine synthetase to prooxidants in human colorectal carcinoma cell lines with different p53 status. Biochem. Pharmacol. 2001, 61, 555–563. [Google Scholar] [CrossRef]







| Compound 2 | Temp 1 (°C) | Time 2 (min) | Yield 3 (%) | Compound 3 | Temp 1 (°C) | Yield 3 (%) | Compound 4 | Yield 3 (%) 1 |
|---|---|---|---|---|---|---|---|---|
| a | 115 | 5 | 72 | a | 160 | 78 | a | 86 |
| b | 115 | 5 | 74 | b | 160 | 37 | b | 90 |
| c | 115 | 5 | 83 | c | 160 | 27 | c | 83 |
| d | 100 | 2 | 61 | d | 140 | 59 | d | 74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loidreau, Y.; Nourrisson, M.-R.; Fruit, C.; Corbière, C.; Marchand, P.; Besson, T. Microwave-Assisted Synthesis of Potential Bioactive Benzo-, Pyrido- or Pyrazino-thieno[3,2-d]pyrimidin-4-amine Analogs of MPC-6827. Pharmaceuticals 2020, 13, 202. https://doi.org/10.3390/ph13090202
Loidreau Y, Nourrisson M-R, Fruit C, Corbière C, Marchand P, Besson T. Microwave-Assisted Synthesis of Potential Bioactive Benzo-, Pyrido- or Pyrazino-thieno[3,2-d]pyrimidin-4-amine Analogs of MPC-6827. Pharmaceuticals. 2020; 13(9):202. https://doi.org/10.3390/ph13090202
Chicago/Turabian StyleLoidreau, Yvonnick, Marie-Renée Nourrisson, Corinne Fruit, Cécile Corbière, Pascal Marchand, and Thierry Besson. 2020. "Microwave-Assisted Synthesis of Potential Bioactive Benzo-, Pyrido- or Pyrazino-thieno[3,2-d]pyrimidin-4-amine Analogs of MPC-6827" Pharmaceuticals 13, no. 9: 202. https://doi.org/10.3390/ph13090202