Biotinylated Quinone as a Chemiluminescence Sensor for Biotin-Avidin Interaction and Biotin Detection Application
Abstract
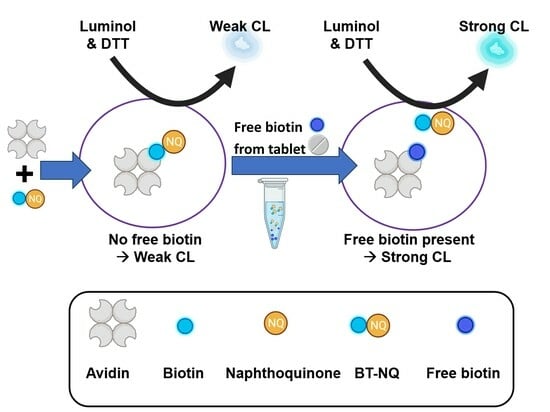
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Instruments
2.3. Synthesis of Biotin-1,2-Naphthoquinone (BT-NQ)
2.4. Calibration Curve of Free Standard Biotin in Competitive Assay
2.5. Detection of Biotin in Supplements
3. Results and Discussions
3.1. BT-NQ Synthesis and Characterization
3.2. Interaction of BT-NQ with Avidin
3.3. Competitive Assay of Biotin Using BT-NQ and Avidin
3.4. Application of the Competitive Assay for Determination of Biotin in Its Supplement Tablet
3.5. Comparison of the Developed Method vs. the Reported Method in the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-15742-1. [Google Scholar]
- Luu Thi Huyen, T.; Vu Thi Nhat, L.; Vu Thi, T.; Le Thi Hong, H. Development of Liquid Chromatography-Mass Spectrometry Method to Determine Biotin Content in Nutritional Products and Supplements. Vietnam J. Food Control 2023, 6, 57–69. [Google Scholar] [CrossRef]
- Mock, D.M.; Fontana, L. Biotin. In Encyclopedia of Human Nutrition; Elsevier: Oxford, UK, 2023; pp. 85–95. [Google Scholar]
- Jungert, A.; Ellinger, S.; Watzl, B.; Richter, M. Revised D-A-CH Reference Values for the Intake of Biotin. Eur. J. Nutr. 2022, 61, 1779–1787. [Google Scholar] [CrossRef]
- Coberly, J. Ask a Pathologist: Biotin Interference. A Cause for Concern? Am. J. Hosp. Med. 2018, 2, 1–2. [Google Scholar] [CrossRef]
- Toshiaki Watanabe, A.F.K.M. Estimated Daily Intake of Biotin in Hospital Meals in Japan through Dietary Surveillance. Trace Nutr. Res. 2013, 30, 64–73. [Google Scholar]
- Yang, J.C.; Jacobs, J.P.; Hwang, M.; Sabui, S.; Liang, F.; Said, H.M.; Skupsky, J. Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype. Nutrients 2023, 15, 264. [Google Scholar] [CrossRef]
- Mock, D.M. Biotin: Physiology, Dietary Sources, and Requirements. In Encyclopedia of Human Nutrition; Elsevier: Oxford, UK, 2013; pp. 182–190. [Google Scholar]
- Biswas, A.; McNamara, C.; Gowda, V.K.; Gala, F.; Sudhakar, S.; Sidpra, J.; Vari, M.S.; Striano, P.; Blaser, S.; Severino, M.; et al. Neuroimaging Features of Biotinidase Deficiency. Am. J. Neuroradiol. 2023, 44, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Berardesca, E.; Fabbrocini, G.; Micali, G.; Tosti, A. Biotin: Overview of the Treatment of Diseases of Cutaneous Appendages and of Hyperseborrhea. G. Ital. Dermatol. Venereol. 2019, 154, 557–566. [Google Scholar] [CrossRef]
- Bender, D.A. Biotin (Vitamin H). In Nutritional Biochemistry of the Vitamins; Cambridge University Press: Cambridge, UK, 2003; pp. 324–344. [Google Scholar]
- Fukui, T.; Iinuma, K.; Oizumi, J.; Izumi, Y. Agar Plate Method Using Lactobacillus Plantarum for Biotin Determination in Serum and Urine. J. Nutr. Sci. Vitaminol. 1994, 40, 491–498. [Google Scholar] [CrossRef]
- Ekpe, A.E.; Hazen, C. Liquid Chromatographic Determination of Biotin in Multivitamin-Multimineral Tablets. J. Pharm. Biomed. Anal. 1998, 16, 1311–1315. [Google Scholar] [CrossRef]
- Thompson, L.B.; Schmitz, D.J.; Pan, S.-J. Determination of Biotin by High-Performance Liquid Chromatography in Infant Formula, Medical Nutritional Products, and Vitamin Premixes. J. AOAC Int. 2006, 89, 1515–1518. [Google Scholar] [CrossRef]
- Livaniou, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Nyalala, J.O.; Ithakissios, D.S.; Evangelatos, G.P. Analytical Techniques for Determining Biotin. J. Chromatogr. A 2000, 881, 331–343. [Google Scholar] [CrossRef]
- Rucker, R.B.; Zempleni, J.; Suttie, J.W.; McCormick, D.B. Handbook of Vitamins; CRC Press: Boca Raton, FL, USA, 2007; Volume 5, ISBN 9780429189050. [Google Scholar]
- Plinton, C.; Mahn, F.P.; Hawrylyshyn, M.; Venturella, V.S.; Senkowski, B.Z. Colorimetric Determination of Biotin. J. Pharm. Sci. 1969, 58, 875–876. [Google Scholar] [CrossRef]
- Fukuda, M.; El-Maghrabey, M.H.; Kishikawa, N.; Ikemoto, K.; Kuroda, N. Ultrasensitive Determination of Pyrroloquinoline Quinone in Human Plasma by HPLC with Chemiluminescence Detection Using the Redox Cycle of Quinone. J. Pharm. Biomed. Anal. 2017, 145, 814–820. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabey, M.; Kishikawa, N.; Kamimura, S.; Ohyama, K.; Kuroda, N. Design of a Dual Functionalized Chemiluminescence Ultrasensitive Probe for Quinones Based on Their Redox Cycle. Application to the Determination of Doxorubicin in Lyophilized Powder and Human Serum. Sens. Actuators B Chem. 2021, 329, 129226. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Sato, Y.; Kaladari, F.; Kishikawa, N.; Kuroda, N. Development of Quinone Linked Immunosorbent Assay (QuLISA) Based on Using Folin’s Reagent as a Non-Enzymatic Tag: Application to Analysis of Food Allergens. Sens. Actuators B Chem. 2022, 368, 132167. [Google Scholar] [CrossRef]
- Kaladari, F.; El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. Development of Signal Multiplication System for Quinone Linked Immunosorbent Assay (Multi-QuLISA) by Using Poly-l-Lysine Dendrigraft and 1,2-Naphthoquinone-4-Sulfonate as Enzyme-Free Tag. Talanta 2023, 253, 123911. [Google Scholar] [CrossRef] [PubMed]
- Kaladari, F.; Kishikawa, N.; Shimada, A.; El-Maghrabey, M.; Kuroda, N. Anthracycline-Functionalized Dextran as a New Signal Multiplication Tagging Approach for Immunoassay. Biosensors 2023, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Guesdon, J.L.; Ternynck, T.; Avrameas, S. The Use of Avidin-Biotin Interaction in Immunoenzymatic Techniques. J. Histochem. Cytochem. 1979, 27, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Biotechnology, P. Instructions HABA 4’-Hydroxyazobenzene-2-Carboxylic Acid; Thermo Scientific: Waltham, MA, USA, 2010. [Google Scholar]
- Büyüktiryaki, S.; Yazıcı, B.; Ersöz, A.; Say, R.; Özkütük, E.B. Application of HRP-Streptavidin Bionanoparticles for Potentiometric Biotin Determination. Bioelectrochemistry 2022, 144, 107993. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Cui, D. Surface Plasmon Resonance Immunoassay for Biotin Determination on a Home-Made Instrument. Procedia Technol. 2017, 27, 87–88. [Google Scholar] [CrossRef]
- Donnenberg, A.D.; Kanias, T.; Triulzi, D.J.; Dennis, C.J.; Meyer, E.M.; Gladwin, M. Improved Quantitative Detection of Biotin-labeled Red Blood Cells by Flow Cytometry. Transfusion 2019, 59, 2691–2698. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Cao, Y.; Tang, Y.; Liu, T. A Study on the Detection of Free and Bound Biotin Based on TR-FRET Technology. Analyst 2022, 147, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Buzid, A.; McGlacken, G.P.; Glennon, J.D.; Luong, J.H.T. Electrochemical Sensing of Biotin Using Nafion-Modified Boron-Doped Diamond Electrode. ACS Omega 2018, 3, 7776–7782. [Google Scholar] [CrossRef] [PubMed]
- Oberbichler, E.; Wiesauer, M.; Schlögl, E.; Stangl, J.; Faschinger, F.; Knör, G.; Gruber, H.J.; Hytönen, V.P. Competitive Binding Assay for Biotin and Biotin Derivatives, Based on Avidin and Biotin-4-Fluorescein. Methods Enzymol. 2020, 633, 1–20. [Google Scholar] [PubMed]









| Studied Concentrations (µg/mL) | Accuracy (Mean% Found) | Precision (RSD, %) |
|---|---|---|
| Intraday | ||
| 1.00 | 98.8 | 2.05 |
| 5.00 | 102 | 0.315 |
| 20.0 | 99.6 | 4.45 |
| 100 | 100 | 1.43 |
| Interday | ||
| 1.00 | 101 | 6.65 |
| 5.00 | 101 | 4.92 |
| 20.0 | 98.0 | 10.6 |
| 100 | 99.4 | 0.969 |
| Conc. Taken (µg/mL) | Amount Found (µg/mL) | % Found a | % RSD (n = 5) |
|---|---|---|---|
| 5.00 | 5.52 | 110 | 8.00 |
| 20.0 | 18.8 | 94.0 | 4.31 |
| 40.0 | 36.5 | 91.3 | 8.67 |
| Method | Sample | LOD | Linear Range | Reference |
|---|---|---|---|---|
| HPLC-FL | Infant formula | – | 0.3–1.0 µM | [14] |
| LC-MS | Nutritional products | 0.01–0.28 µM | 0.03–9.2 µM | [2] |
| HPLC-UV | Biotin supplements | – | 3.3–13.2 µM | [13] |
| Affinity-based potentiometry | Infant formula and collagen hyaluronic | 0.3 FM | 1–100 fM | [25] |
| Electrochemical sensing | Blood plasma | 5 nM | – | [29] |
| TR-FRET assay | Solution containing biotin | 0.03 nM | 0.05–100 nM | [28] |
| SPR analytical system | Biotin derivatives | 0.66 µM | 0.66–6600 µM | [26] |
| Proximity assay | Biotin supplements | 0.58 µM | 1.0–100 µM | BT-NQ assay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaladari, F.; El-Maghrabey, M.; Kawazato, M.; Kishikawa, N.; Kuroda, N. Biotinylated Quinone as a Chemiluminescence Sensor for Biotin-Avidin Interaction and Biotin Detection Application. Sensors 2023, 23, 9611. https://doi.org/10.3390/s23239611
Kaladari F, El-Maghrabey M, Kawazato M, Kishikawa N, Kuroda N. Biotinylated Quinone as a Chemiluminescence Sensor for Biotin-Avidin Interaction and Biotin Detection Application. Sensors. 2023; 23(23):9611. https://doi.org/10.3390/s23239611
Chicago/Turabian StyleKaladari, Fatema, Mahmoud El-Maghrabey, Megumi Kawazato, Naoya Kishikawa, and Naotaka Kuroda. 2023. "Biotinylated Quinone as a Chemiluminescence Sensor for Biotin-Avidin Interaction and Biotin Detection Application" Sensors 23, no. 23: 9611. https://doi.org/10.3390/s23239611